A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa
- PMID: 35008864
- PMCID: PMC8745769
- DOI: 10.3390/ijms23010439
A Fiber-Rich Diet and Radiation-Induced Injury in the Murine Intestinal Mucosa
Abstract
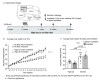
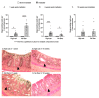
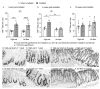
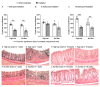
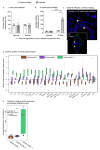
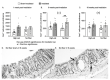
Dietary fiber is considered a strong intestinal protector, but we do not know whether dietary fiber protects against the long-lasting mucosal damage caused by ionizing radiation. To evaluate whether a fiber-rich diet can ameliorate the long-lasting pathophysiological hallmarks of the irradiated mucosa, C57BL/6J mice on a fiber-rich bioprocessed oat bran diet or a fiber-free diet received 32 Gray in four fractions to the distal colorectum using a linear accelerator and continued on the diets for one, six or 18 weeks. We quantified degenerating crypts, crypt fission, cell proliferation, crypt survival, macrophage density and bacterial infiltration. Crypt loss through crypt degeneration only occurred in the irradiated mice. Initially, it was most frequent in the fiber-deprived group but declined to levels similar to the fiber-consuming group by 18 weeks. The fiber-consuming group had a fast response to irradiation, with crypt fission for growth or healing peaking already at one week post-irradiation, while crypt fission in the fiber-deprived group peaked at six weeks. A fiber-rich diet allowed for a more intense crypt cell proliferation, but the recovery of crypts was eventually lost by 18 weeks. Bacterial infiltration was a late phenomenon, evident in the fiber-deprived animals and intensified manyfold after irradiation. Bacterial infiltration also coincided with a specific pro-inflammatory serum cytokine profile. In contrast, mice on a fiber-rich diet were completely protected from irradiation-induced bacterial infiltration and exhibited a similar serum cytokine profile as sham-irradiated mice on a fiber-rich diet. Our findings provide ample evidence that dietary fiber consumption modifies the onset, timing and intensity of radiation-induced pathophysiological processes in the intestinal mucosa. However, we need more knowledge, not least from clinical studies, before this finding can be introduced to a new and refined clinical practice.
Keywords: bacteria; colon; cytokines; dietary fiber; intestine; irradiation; oat.
Conflict of interest statement
A. Rascón is a part-time employee of Glucanova, the provider of the bioprocessed oat bran. She is also the author of the patent for its preparation.
Figures

References
-
- Steineck G., Skokic V., Sjöberg F., Bull C., Alevronta E., Dunberger G., Bergmark K., Wilderäng U., Oh J.H., Deasy J.O., et al. Identifying radiation-induced survivorship syndromes affecting bowel health in a cohort of gynecological cancer survivors. PLoS ONE. 2017;12:e0171461. doi: 10.1371/journal.pone.0171461. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

