Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism
- PMID: 35008868
- PMCID: PMC8745631
- DOI: 10.3390/ijms23010440
Novel Purine Derivative ITH15004 Facilitates Exocytosis through a Mitochondrial Calcium-Mediated Mechanism
Abstract
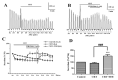
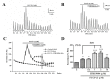
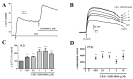
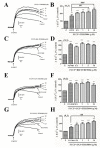
Upon depolarization of chromaffin cells (CCs), a prompt release of catecholamines occurs. This event is triggered by a subplasmalemmal high-Ca2+ microdomain (HCMD) generated by Ca2+ entry through nearby voltage-activated calcium channels. HCMD is efficiently cleared by local mitochondria that avidly take up Ca2+ through their uniporter (MICU), then released back to the cytosol through mitochondrial Na+/Ca2+ exchanger (MNCX). We found that newly synthesized derivative ITH15004 facilitated the release of catecholamines triggered from high K+-depolarized bovine CCs. Such effect seemed to be due to regulation of mitochondrial Ca2+ circulation because: (i) FCCP-potentiated secretory responses decay was prevented by ITH15004; (ii) combination of FCCP and ITH15004 exerted additive secretion potentiation; (iii) such additive potentiation was dissipated by the MICU blocker ruthenium red (RR) or the MNCX blocker CGP37157 (CGP); (iv) combination of FCCP and ITH15004 produced both additive augmentation of cytosolic Ca2+ concentrations ([Ca2+]c) K+-challenged BCCs, and (v) non-inactivated [Ca2+]c transient when exposed to RR or CGP. On pharmacological grounds, data suggest that ITH15004 facilitates exocytosis by acting on mitochondria-controlled Ca2+ handling during K+ depolarization. These observations clearly show that ITH15004 is a novel pharmacological tool to study the role of mitochondria in the regulation of the bioenergetics and exocytosis in excitable cells.
Keywords: ITH15004; calcium signalling; catecholamine release; chromaffin cell; mitochondria; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Hydrogen sulphide facilitates exocytosis by regulating the handling of intracellular calcium by chromaffin cells.Pflugers Arch. 2018 Aug;470(8):1255-1270. doi: 10.1007/s00424-018-2147-7. Epub 2018 May 2. Pflugers Arch. 2018. PMID: 29721607
-
The quantal catecholamine release from mouse chromaffin cells challenged with repeated ACh pulses is regulated by the mitochondrial Na+ /Ca2+ exchanger.J Physiol. 2017 Mar 15;595(6):2129-2146. doi: 10.1113/JP273339. Epub 2017 Feb 7. J Physiol. 2017. PMID: 27982456 Free PMC article.
-
Novel features on the regulation by mitochondria of calcium and secretion transients in chromaffin cells challenged with acetylcholine at 37°C.Physiol Rep. 2013 Dec 19;1(7):e00182. doi: 10.1002/phy2.182. eCollection 2013 Dec 1. Physiol Rep. 2013. PMID: 24744861 Free PMC article.
-
Mitochondria and chromaffin cell function.Pflugers Arch. 2012 Jul;464(1):33-41. doi: 10.1007/s00424-012-1074-2. Epub 2012 Jan 27. Pflugers Arch. 2012. PMID: 22278417 Review.
-
Calcium entry, calcium redistribution, and exocytosis.Ann N Y Acad Sci. 2002 Oct;971:108-16. doi: 10.1111/j.1749-6632.2002.tb04444.x. Ann N Y Acad Sci. 2002. PMID: 12438100 Review.
Cited by
-
The Arabidopsis RTH plays an important role in regulation of iron (Fe) absorption and transport.Plant Cell Rep. 2024 Apr 30;43(5):133. doi: 10.1007/s00299-024-03214-x. Plant Cell Rep. 2024. PMID: 38687356
References
-
- Carbone E., Borges R., Eiden L.E., Garcia A.G., Hernandez-Cruz A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019;9:1443–1502. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

