TGF-β1 Potentiates the Cytotoxicity of Cadmium by Induction of a Metal Transporter, ZIP8, Mediated by the ALK5-Smad2/3 and ALK5-Smad3-p38 MAPK Signal Pathways in Cultured Vascular Endothelial Cells
- PMID: 35008873
- PMCID: PMC8745387
- DOI: 10.3390/ijms23010448
TGF-β1 Potentiates the Cytotoxicity of Cadmium by Induction of a Metal Transporter, ZIP8, Mediated by the ALK5-Smad2/3 and ALK5-Smad3-p38 MAPK Signal Pathways in Cultured Vascular Endothelial Cells
Abstract
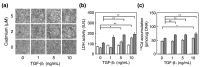
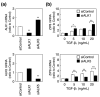
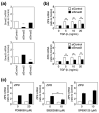
Vascular endothelial cells cover the luminal surface of blood vessels in a monolayer and play a role in the regulation of vascular functions, such as the blood coagulation-fibrinolytic system. When the monolayer is severely or repeatedly injured, platelets aggregate at the damaged site and release transforming growth factor (TGF)-β1 in large quantities from their α-granules. Cadmium is a heavy metal that is toxic to various organs, including the kidneys, bones, liver, and blood vessels. Our previous study showed that the expression level of Zrt/Irt-related protein 8 (ZIP8), a metal transporter that transports cadmium from the extracellular fluid into the cytosol, is a crucial factor in determining the sensitivity of vascular endothelial cells to cadmium cytotoxicity. In the present study, TGF-β1 was discovered to potentiate cadmium-induced cytotoxicity by increasing the intracellular accumulation of cadmium in cells. Additionally, TGF-β1 induced the expression of ZIP8 via the activin receptor-like kinase 5-Smad2/3 signaling pathways; Smad3-mediated induction of ZIP8 was associated with or without p38 mitogen-activated protein kinase (MAPK). These results suggest that the cytotoxicity of cadmium to vascular endothelial cells increases when damaged endothelial monolayers that are highly exposed to TGF-β1 are repaired.
Keywords: Smad2/3; Zrt- and Irt-like protein transporter; cadmium; endothelial cell; p38 MAPK; transforming growth factor-β1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

