Pulsed Electric Fields Alter Expression of NF-κB Promoter-Controlled Gene
- PMID: 35008875
- PMCID: PMC8745616
- DOI: 10.3390/ijms23010451
Pulsed Electric Fields Alter Expression of NF-κB Promoter-Controlled Gene
Abstract
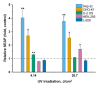
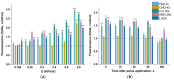
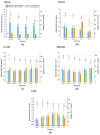
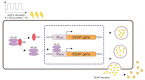
The possibility to artificially adjust and fine-tune gene expression is one of the key milestones in bioengineering, synthetic biology, and advanced medicine. Since the effects of proteins or other transgene products depend on the dosage, controlled gene expression is required for any applications, where even slight fluctuations of the transgene product impact its function or other critical cell parameters. In this context, physical techniques demonstrate optimistic perspectives, and pulsed electric field technology is a potential candidate for a noninvasive, biophysical gene regulator, exploiting an easily adjustable pulse generating device. We exposed mammalian cells, transfected with a NF-κB pathway-controlled transcription system, to a range of microsecond-duration pulsed electric field parameters. To prevent toxicity, we used protocols that would generate relatively mild physical stimulation. The present study, for the first time, proves the principle that microsecond-duration pulsed electric fields can alter single-gene expression in plasmid context in mammalian cells without significant damage to cell integrity or viability. Gene expression might be upregulated or downregulated depending on the cell line and parameters applied. This noninvasive, ligand-, cofactor-, nanoparticle-free approach enables easily controlled direct electrostimulation of the construct carrying the gene of interest; the discovery may contribute towards the path of simplification of the complexity of physical systems in gene regulation and create further synergies between electronics, synthetic biology, and medicine.
Keywords: NF-κB; cell line; inducible gene transcription control; mammalian cells; microsecond pulsed electric field; reporter assay; secreted alkaline phosphatase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Stuible M., Burlacu A., Perret S., Brochu D., Paul-Roc B., Baardsnes J., Loignon M., Grazzini E., Durocher Y. Optimization of a high-cell-density polyethylenimine transfection method for rapid protein production in CHO-EBNA1 cells. J. Biotechnol. 2018;281:39–47. doi: 10.1016/j.jbiotec.2018.06.307. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

