Oxidized Substrates of APEH as a Tool to Study the Endoprotease Activity of the Enzyme
- PMID: 35008880
- PMCID: PMC8745263
- DOI: 10.3390/ijms23010443
Oxidized Substrates of APEH as a Tool to Study the Endoprotease Activity of the Enzyme
Abstract
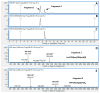
APEH is a ubiquitous and cytosolic serine protease belonging to the prolyl oligopeptidase (POP) family, playing a critical role in the processes of degradation of proteins through both exo- and endopeptidase events. Endopeptidase activity has been associated with protein oxidation; however, the actual mechanisms have yet to be elucidated. We show that a synthetic fragment of GDF11 spanning the region 48-64 acquires sensitivity to the endopeptidase activity of APEH only when the methionines are transformed into the corresponding sulphoxide derivatives. The data suggest that the presence of sulphoxide-modified methionines is an important prerequisite for the substrates to be processed by APEH and that the residue is crucial for switching the enzyme activity from exo- to endoprotease. The cleavage occurs on residues placed on the C-terminal side of Met(O), with an efficiency depending on the methionine adjacent residues, which thereby may play a crucial role in driving and modulating APEH endoprotease activity.
Keywords: APEH; endoproteolytic activity; oxidative stress; oxidized methionine; oxidized substrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
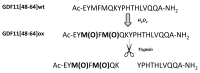


References
-
- Garcia-Rojo G., Gamiz F., Ampuero E., Rojas-Espina D., Sandoval R., Rozas C., Morales B., Wyneken U., Pancetti F. In Vivo Sub-chronic Treatment with Dichlorvos in Young Rats Promotes Synaptic Plasticity and Learning by a Mechanism that Involves Acylpeptide Hydrolase Instead of Acetylcholinesterase Inhibition. Correlation with Endogenous beta-Amyloid Levels. Front. Pharmacol. 2017;8:483. doi: 10.3389/fphar.2017.00483. - DOI - PMC - PubMed
-
- Fujino T., Tada T., Beppu M., Kikugawa K. Purification and characterization of a serine protease in erythrocyte cytosol that is adherent to oxidized membranes and preferentially degrades proteins modified by oxidation and glycation. J. Biochem. 1998;124:1077–1085. doi: 10.1093/oxfordjournals.jbchem.a022224. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- Development of novel therapeutic approaches for treatment of resistant neoplastic diseases (SATIN)/Regione Campania
- NANOCAN, NANOfotonica per la lotta al CANcro/Regione Campania
- Fighting Cancer resistance: Multidisciplinary integrated Platform for a technological Innovative Approach to Oncotherapies (Campania Oncotherapies)/Regione Campania
- Research Project on CAR T cells for hematological malignancies and solid tumors/Ministero della Salute
- F/200050/01-03/X45 - Sviluppo di piattaforme molecolari e cellulari per l'identificazione di prodotti innovativi ad attività NUTRAceutica da Biotrasformazioni mediante organismi ESTremofili (NUTRABEST)/Ministry of Economic Development
LinkOut - more resources
Full Text Sources
Miscellaneous

