Relevance of a Novel Circuit-Level Model of Episodic Memories to Alzheimer's Disease
- PMID: 35008886
- PMCID: PMC8745479
- DOI: 10.3390/ijms23010462
Relevance of a Novel Circuit-Level Model of Episodic Memories to Alzheimer's Disease
Abstract
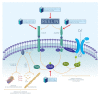
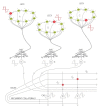
The medial temporal lobe memory system has long been identified as the brain region showing the first histopathological changes in early Alzheimer's disease (AD), and the functional decline observed in patients also points to a loss of function in this brain area. Nonetheless, the exact identity of the neurons and networks that undergo deterioration has not been determined so far. A recent study has identified the entorhinal and hippocampal neural circuits responsible for encoding new episodic memories. Using this novel model we describe the elements of the episodic memory network that are especially vulnerable in early AD. We provide a hypothesis of how reduced reelin signaling within such a network can promote AD-related changes. Establishing novel associations and creating a temporal structure for new episodic memories are both affected in AD. Here, we furnish a reasonable explanation for both of these previous observations.
Keywords: Alzheimer’s disease; EPISODE module; anterograde amnesia; entorhinal cortex; episodic memories; granule cells; hippocampus; neurogenesis; reelin; synaptogenesis.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Episodic memory on the path to Alzheimer's disease.Curr Opin Neurobiol. 2011 Dec;21(6):929-34. doi: 10.1016/j.conb.2011.10.021. Epub 2011 Nov 11. Curr Opin Neurobiol. 2011. PMID: 22079495 Free PMC article. Review.
-
Content-specific vulnerability of recent episodic memories in Alzheimer's disease.Neuropsychologia. 2021 Sep 17;160:107976. doi: 10.1016/j.neuropsychologia.2021.107976. Epub 2021 Jul 24. Neuropsychologia. 2021. PMID: 34314781 Free PMC article.
-
Different Temporal Patterns of Specific and General Autobiographical Memories across the Lifespan in Alzheimer's Disease.Behav Neurol. 2015;2015:963460. doi: 10.1155/2015/963460. Epub 2015 Jun 15. Behav Neurol. 2015. PMID: 26175549 Free PMC article.
-
Retrieval of autobiographical memory in Alzheimer's disease: relation to volumes of medial temporal lobe and other structures.Hippocampus. 2005;15(4):535-50. doi: 10.1002/hipo.20090. Hippocampus. 2005. PMID: 15884035
-
Thalamic pathology and memory loss in early Alzheimer's disease: moving the focus from the medial temporal lobe to Papez circuit.Brain. 2016 Jul;139(Pt 7):1877-90. doi: 10.1093/brain/aww083. Epub 2016 Apr 28. Brain. 2016. PMID: 27190025 Free PMC article. Review.
Cited by
-
Cholecystokinin B receptor agonists alleviates anterograde amnesia in cholecystokinin-deficient and aged Alzheimer's disease mice.Alzheimers Res Ther. 2024 May 15;16(1):109. doi: 10.1186/s13195-024-01472-1. Alzheimers Res Ther. 2024. PMID: 38750512 Free PMC article.
-
Beneficial Effects of Echinacoside on Cognitive Impairment and Diabetes in Type 2 Diabetic db/db Mice.Exp Clin Endocrinol Diabetes. 2024 Aug;132(8):420-430. doi: 10.1055/a-2298-4593. Epub 2024 Apr 3. Exp Clin Endocrinol Diabetes. 2024. PMID: 38569512 Free PMC article.
References
-
- Vogel J.W., Iturria-Medina Y., Strandberg O.T., Smith R., Levitis E., Evans A.C., Hansson O., Alzheimer’s Disease Neuroimaging Initiative. the Swedish BioFinder Study Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat. Commun. 2020;11:2612. doi: 10.1038/s41467-020-15701-2. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

