MTEP, a Selective mGluR5 Antagonist, Had a Neuroprotective Effect but Did Not Prevent the Development of Spontaneous Recurrent Seizures and Behavioral Comorbidities in the Rat Lithium-Pilocarpine Model of Epilepsy
- PMID: 35008924
- PMCID: PMC8745728
- DOI: 10.3390/ijms23010497
MTEP, a Selective mGluR5 Antagonist, Had a Neuroprotective Effect but Did Not Prevent the Development of Spontaneous Recurrent Seizures and Behavioral Comorbidities in the Rat Lithium-Pilocarpine Model of Epilepsy
Abstract
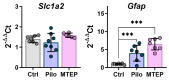
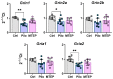
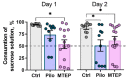
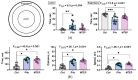
Metabotropic glutamate receptors (mGluRs) are expressed predominantly on neurons and glial cells and are involved in the modulation of a wide range of signal transduction cascades. Therefore, different subtypes of mGluRs are considered a promising target for the treatment of various brain diseases. Previous studies have demonstrated the seizure-induced upregulation of mGluR5; however, its functional significance is still unclear. In the present study, we aimed to clarify the effect of treatment with the selective mGluR5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP) on epileptogenesis and behavioral impairments in rats using the lithium-pilocarpine model. We found that the administration of MTEP during the latent phase of the model did not improve survival, prevent the development of epilepsy, or attenuate its manifestations in rats. However, MTEP treatment completely prevented neuronal loss and partially attenuated astrogliosis in the hippocampus. An increase in excitatory amino acid transporter 2 expression, which has been detected in treated rats, may prevent excitotoxicity and be a potential mechanism of neuroprotection. We also found that MTEP administration did not prevent the behavioral comorbidities such as depressive-like behavior, motor hyperactivity, reduction of exploratory behavior, and cognitive impairments typical in the lithium-pilocarpine model. Thus, despite the distinct neuroprotective effect, the MTEP treatment was ineffective in preventing epilepsy.
Keywords: excitatory amino acid transporter 2; glial fibrillary acidic protein; hippocampus; immunohistochemistry; neuronal loss; novel object recognition test; open field test; temporal lobe epilepsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Neuroprotective potential of mGluR5 antagonist MTEP: effects on kainate-induced excitotoxicity in the rat hippocampus.Pharmacol Rep. 2010 Nov-Dec;62(6):1051-61. doi: 10.1016/s1734-1140(10)70367-4. Pharmacol Rep. 2010. PMID: 21273662
-
Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors.Br J Pharmacol. 2005 Jun;145(4):527-34. doi: 10.1038/sj.bjp.0706219. Br J Pharmacol. 2005. PMID: 15821750 Free PMC article.
-
mGluR5 antagonist-induced psychoactive properties: MTEP drug discrimination, a pharmacologically selective non-NMDA effect with apparent lack of reinforcing properties.J Pharmacol Exp Ther. 2014 Apr;349(1):155-64. doi: 10.1124/jpet.113.211185. Epub 2014 Jan 28. J Pharmacol Exp Ther. 2014. PMID: 24472725
-
Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP.CNS Drug Rev. 2006 Summer;12(2):149-66. doi: 10.1111/j.1527-3458.2006.00149.x. CNS Drug Rev. 2006. PMID: 16958988 Free PMC article. Review.
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
Cited by
-
Changes in Metabotropic Glutamate Receptor Gene Expression in Rat Brain in a Lithium-Pilocarpine Model of Temporal Lobe Epilepsy.Int J Mol Sci. 2022 Mar 2;23(5):2752. doi: 10.3390/ijms23052752. Int J Mol Sci. 2022. PMID: 35269897 Free PMC article.
-
Ameliorative Potential of (-) Pseudosemiglabrin in Mice with Pilocarpine-Induced Epilepsy: Antioxidant, Anti-Inflammatory, Anti-Apoptotic, and Neurotransmission Modulation.Int J Mol Sci. 2023 Jun 28;24(13):10773. doi: 10.3390/ijms241310773. Int J Mol Sci. 2023. PMID: 37445950 Free PMC article.
-
Exploring the material basis and molecular targets of Changma Xifeng tablet in treating Tourette syndrome: an integrative approach of network pharmacology and miRNA analysis.Metab Brain Dis. 2024 Dec;39(8):1573-1590. doi: 10.1007/s11011-024-01408-6. Epub 2024 Oct 22. Metab Brain Dis. 2024. PMID: 39436634
-
Molecular mechanisms of excitotoxicity and their relevance to the pathogenesis of neurodegenerative diseases-an update.Acta Pharmacol Sin. 2025 May 19. doi: 10.1038/s41401-025-01576-w. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40389567 Review.
-
Antiepileptogenic Effects of Anakinra, Lamotrigine and Their Combination in a Lithium-Pilocarpine Model of Temporal Lobe Epilepsy in Rats.Int J Mol Sci. 2023 Oct 20;24(20):15400. doi: 10.3390/ijms242015400. Int J Mol Sci. 2023. PMID: 37895080 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

