Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies
- PMID: 35008938
- PMCID: PMC8745567
- DOI: 10.3390/ijms23010512
Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies
Abstract
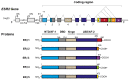
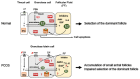
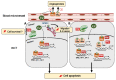
Estradiol (E2) is a major hormone controlling women fertility, in particular folliculogenesis. This steroid, which is locally produced by granulosa cells (GC) within ovarian follicles, controls the development and selection of dominant preovulatory follicles. E2 effects rely on a complex set of nuclear and extra-nuclear signal transduction pathways principally triggered by its nuclear receptors, ERα and ERβ. These transcription factors are differentially expressed within follicles, with ERβ being the predominant ER in GC. Several ERβ splice isoforms have been identified and display specific structural features, which greatly complicates the nature of ERβ-mediated E2 signaling. This review aims at providing a concise overview of the main actions of E2 during follicular growth, maturation, and selection in human. It also describes the current understanding of the various roles of ERβ splice isoforms, especially their influence on cell fate. We finally discuss how E2 signaling deregulation could participate in two ovarian pathogeneses characterized by either a follicular arrest, as in polycystic ovary syndrome, or an excess of GC survival and proliferation, leading to granulosa cell tumors. This review emphasizes the need for further research to better understand the molecular basis of E2 signaling throughout folliculogenesis and to improve the efficiency of ovarian-related disease therapies.
Keywords: estradiol; estrogen receptors isoforms; folliculogenesis; granulosa cell tumors; granulosa cells; polycystic ovary syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McNatty K.P., Moore Smith D., Osathanondh R., Ryan K.J. The human antral follicle: Functional correlates of growth and atresia. Ann. Biol. Anim. Bioch. Biophys. 1979;19:1547–1558. doi: 10.1051/rnd:19790916. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

