Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms
- PMID: 35008974
- PMCID: PMC8745555
- DOI: 10.3390/ijms23010545
Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms
Abstract
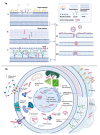
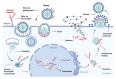
Despite the great strides in healthcare during the last century, some challenges still remained unanswered. The development of multi-drug resistant bacteria, the alarming growth of fungal infections, the emerging/re-emerging of viral diseases are yet a worldwide threat. Since the discovery of natural antimicrobial peptides able to broadly hit several pathogens, peptide-based therapeutics have been under the lenses of the researchers. This review aims to focus on synthetic peptides and elucidate their multifaceted mechanisms of action as antiviral, antibacterial and antifungal agents. Antimicrobial peptides generally affect highly preserved structures, e.g., the phospholipid membrane via pore formation or other constitutive targets like peptidoglycans in Gram-negative and Gram-positive bacteria, and glucan in the fungal cell wall. Additionally, some peptides are particularly active on biofilm destabilizing the microbial communities. They can also act intracellularly, e.g., on protein biosynthesis or DNA replication. Their intracellular properties are extended upon viral infection since peptides can influence several steps along the virus life cycle starting from viral receptor-cell interaction to the budding. Besides their mode of action, improvements in manufacturing to increase their half-life and performances are also taken into consideration together with advantages and impairments in the clinical usage. Thus far, the progress of new synthetic peptide-based approaches is making them a promising tool to counteract emerging infections.
Keywords: antibacterial; antifungal; antimicrobial peptides; antiviral; peptide-based therapies; synthetic peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fleming A., Allison V.D. Observations on a Bacteriolytic Substance (“Lysozyme”) Found in Secretions and Tissues. Br. J. Exp. Pathol. 1922;3:252–260.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

