Thymosin β4 Is an Endogenous Iron Chelator and Molecular Switcher of Ferroptosis
- PMID: 35008976
- PMCID: PMC8745404
- DOI: 10.3390/ijms23010551
Thymosin β4 Is an Endogenous Iron Chelator and Molecular Switcher of Ferroptosis
Abstract
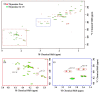
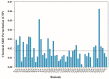
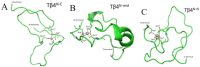
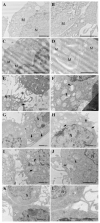
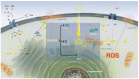
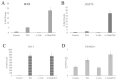
Thymosin β4 (Tβ4) was extracted forty years agofrom calf thymus. Since then, it has been identified as a G-actin binding protein involved in blood clotting, tissue regeneration, angiogenesis, and anti-inflammatory processes. Tβ4 has also been implicated in tumor metastasis and neurodegeneration. However, the precise roles and mechanism(s) of action of Tβ4 in these processes remain largely unknown, with the binding of the G-actin protein being insufficient to explain these multi-actions. Here we identify for the first time the important role of Tβ4 mechanism in ferroptosis, an iron-dependent form of cell death, which leads to neurodegeneration and somehow protects cancer cells against cell death. Specifically, we demonstrate four iron2+ and iron3+ binding regions along the peptide and show that the presence of Tβ4 in cell growing medium inhibits erastin and glutamate-induced ferroptosis in the macrophage cell line. Moreover, Tβ4 increases the expression of oxidative stress-related genes, namely BAX, hem oxygenase-1, heat shock protein 70 and thioredoxin reductase 1, which are downregulated during ferroptosis. We state the hypothesis that Tβ4 is an endogenous iron chelator and take part in iron homeostasis in the ferroptosis process. We discuss the literature data of parallel involvement of Tβ4 and ferroptosis in different human pathologies, mainly cancer and neurodegeneration. Our findings confronted with literature data show that controlled Tβ4 release could command on/off switching of ferroptosis and may provide novel therapeutic opportunities in cancer and tissue degeneration pathologies.
Keywords: NMR; TEM; ferroptosis; mRNA; metal chelation; molecular dynamics; thymosine beta 4.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

