A Glutathione Peroxidase Gene from Litopenaeus vannamei Is Involved in Oxidative Stress Responses and Pathogen Infection Resistance
- PMID: 35008992
- PMCID: PMC8745291
- DOI: 10.3390/ijms23010567
A Glutathione Peroxidase Gene from Litopenaeus vannamei Is Involved in Oxidative Stress Responses and Pathogen Infection Resistance
Abstract
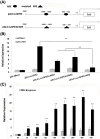
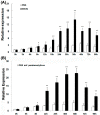
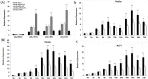
In shrimp, several glutathione peroxidase (GPX) genes have been cloned and functionally studied. Increasing evidence suggests the genes' involvement in white spot syndrome virus (WSSV)- or Vibrio alginolyticus-infection resistance. In the present study, a novel GXP gene (LvGPX3) was cloned in Litopenaeus vannamei. Promoter of LvGPX3 was activated by NF-E2-related factor 2. Further study showed that LvGPX3 expression was evidently accelerated by oxidative stress or WSSV or V. alginolyticus infection. Consistently, downregulated expression of LvGPX3 increased the cumulative mortality of WSSV- or V. alginolyticus-infected shrimp. Similar results occurred in shrimp suffering from oxidative stress. Moreover, LvGPX3 was important for enhancing Antimicrobial peptide (AMP) gene expression in S2 cells with lipopolysaccharide treatment. Further, knockdown of LvGPX3 expression significantly suppressed expression of AMPs, such as Penaeidins 2a, Penaeidins 3a and anti-lipopolysaccharide factor 1 in shrimp. AMPs have been proven to be engaged in shrimp WSSV- or V. alginolyticus-infection resistance; it was inferred that LvGPX3 might enhance shrimp immune response under immune challenges, such as increasing expression of AMPs. The regulation mechanism remains to be further studied.
Keywords: Antimicrobial peptide genes; Litopenaeus vannamei; glutathione peroxidase; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Koeberle S.C., Gollowitzer A., Laoukili J., Kranenburg O., Werz O., Koeberle A., Kipp A. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms. Redox Biol. 2020;28:101338. doi: 10.1016/j.redox.2019.101388. - DOI - PMC - PubMed
-
- Borchert A., Kalms J., Roth S.R., Rademacher M., Schmidt A., Holzhutter H.-G., Kühn H., Scheerer P. Crystal structure and functional characterization of selenocysteine-containing glutathione peroxidase 4 suggests an alternative mechanism of peroxide reduction. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2018;1863:1095–1107. doi: 10.1016/j.bbalip.2018.06.006. - DOI - PubMed
-
- Rojas J., Díaz A.A.B. Chapter 1-Antioxidant Activity of Phenolic Compounds Biosynthesized by Plants and Its Relationship With Prevention of Neurodegenerative Diseases—ScienceDirect. Bioact. Compd. 2019:3–31. doi: 10.1016/B978-0-12-814774-0.00001-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

