Arabidopsis thaliana Plant Natriuretic Peptide Active Domain Forms Amyloid-like Fibrils in a pH-Dependent Manner
- PMID: 35009013
- PMCID: PMC8747288
- DOI: 10.3390/plants11010009
Arabidopsis thaliana Plant Natriuretic Peptide Active Domain Forms Amyloid-like Fibrils in a pH-Dependent Manner
Abstract
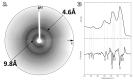
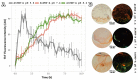
Plant natriuretic peptides (PNPs) are hormones that have been extracted from many different species, with the Arabidopsis thaliana PNP (AtPNP-A) being the most studied among them. AtPNP-A is a signaling molecule that consists of 130 residues and is secreted into the apoplast, under conditions of biotic or abiotic stress. AtPNP-A has distant sequence homology with human ANP, a protein that forms amyloid fibrils in vivo. In this work, we investigated the amyloidogenic properties of a 34-residue-long peptide, located within the AtPNP-A sequence, in three different pH conditions, using transmission electron microscopy, X-ray fiber diffraction, ATR FT-IR spectroscopy, Congo red and Thioflavin T staining assays. We also utilize bioinformatics tools to study its association with known plant amyloidogenic proteins and other A. thaliana proteins. Our results reveal a new case of a pH-dependent amyloid forming peptide in A. thaliana, with a potential functional role.
Keywords: Arabidopsis thaliana; amyloid fibrils; functional amyloid; natriuretic peptides; plant natriuretic peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

