Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration
- PMID: 35009069
- PMCID: PMC8747280
- DOI: 10.3390/plants11010065
Guard-Cell-Specific Expression of Phototropin2 C-Terminal Fragment Enhances Leaf Transpiration
Abstract
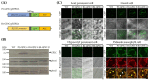
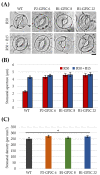
Phototropins (phot1 and phot2) are plant-specific blue light receptors that mediate chloroplast movement, stomatal opening, and phototropism. Phototropin is composed of the N-terminus LOV1 and LOV2 domains and the C-terminus Ser/Thr kinase domain. In previous studies, 35-P2CG transgenic plants expressing the phot2 C-terminal fragment-GFP fusion protein (P2CG) under the control of 35S promoter showed constitutive phot2 responses, including chloroplast avoidance response, stomatal opening, and reduced hypocotyl phototropism regardless of blue light, and some detrimental growth phenotypes. In this study, to exclude the detrimental growth phenotypes caused by the ectopic expression of P2C and to improve leaf transpiration, we used the PHOT2 promoter for the endogenous expression of GFP-fused P2C (GP2C) (P2-GP2C) and the BLUS1 promoter for the guard-cell-specific expression of GP2C (B1-GP2C), respectively. In P2-GP2C plants, GP2C expression induced constitutive phototropin responses and a relatively dwarf phenotype as in 35-P2CG plants. In contrast, B1-GP2C plants showed the guard-cell-specific P2C expression that induced constitutive stomatal opening with normal phototropism, chloroplast movement, and growth phenotype. Interestingly, leaf transpiration was significantly improved in B1-GP2C plants compared to that in P2-GP2C plants and WT. Taken together, this transgenic approach could be applied to improve leaf transpiration in indoor plants.
Keywords: Arabidopsis thaliana; BLUS1 (BLUE LIGHT SIGNALING1); phototropin2; stomatal opening; transpiration.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
LinkOut - more resources
Full Text Sources

