Expression Analysis of Phenylpropanoid Pathway Genes and Metabolomic Analysis of Phenylpropanoid Compounds in Adventitious, Hairy, and Seedling Roots of Tartary Buckwheat
- PMID: 35009093
- PMCID: PMC8747410
- DOI: 10.3390/plants11010090
Expression Analysis of Phenylpropanoid Pathway Genes and Metabolomic Analysis of Phenylpropanoid Compounds in Adventitious, Hairy, and Seedling Roots of Tartary Buckwheat
Abstract
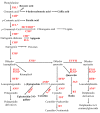
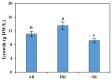
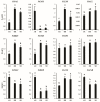
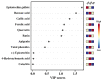
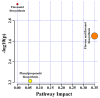
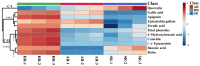
Tartary buckwheat (Fagopyrum tataricum) is an important crop that belongs to the Polygonaceae family, whose roots have received considerable attention due to the presence of compounds with high nutritional and medicinal value. In this study, we aimed to develop an efficient protocol for the culture of adventitious (ARs) and hairy (HRs) roots on a half-strength Schenk and Hildebrandt (SH) medium containing different concentrations of the auxins, α-naphthaleneacetic acid (NAA), indole-3-butyric acid (IBA), and indole-3-acetic acid (IAA). The highest percentage of root induction (91.67%) was achieved with 0.5 mg/L IAA, whereas the greatest number of roots was found in 1 mg/L IAA. In contrast, 0.1 mg/L IBA returned the longest roots. As expected, HRs were obtained from in vitro leaf explants infected with Agrobacterium rhizogenes R1000. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of 11 phenolic pathway genes revealed that five genes (FtPAL, FtC3H, FtHQT, FtCHS, and FtANS) were highly expressed in HRs, whereas only four (FtC4H, FtFLS2, FtDFR, and FtANR), and three (Ft4CL, FtCHI, and FtF3H) were recognized in the ARs and seedling roots (SRs), respectively. HPLC analysis of phenolic compounds in different root cultures showed that the majority of the phenolic compounds (both individual and total) were significantly accumulated in the HRs. Principal component analysis (PCA) identified differences among the three root types, whereby HRs were separated from ARs and SRs based on the amount of phenolic compounds present. Analysis of the metabolic pathway revealed that among the identified metabolites, the 3, 2, and 1 pathways were associated with flavonoid, flavone and flavonol, and phenylpropanoid biosynthesis, respectively. Hierarchical clustering analysis and the heat map showed that the different root cultures presented unique metabolites.
Keywords: Agrobacterium rhizogenes; Fagopyrum tataricum; auxins; buckwheat; phenolic compounds; root culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Guillon S., Trémouillaux-Guiller J., Pati P.K., Gantet P. Hairy roots: A powerful tool for plant biotechnological advances. In: Ramawat K.G., Merillon J.M., editors. Bioactive Molecules and Medicinal Plants. Springer; Berlin/Heidelberg, Germany: 2008. pp. 271–283.
-
- Bhatia S., Bera T., Dahiya R., Bera T., Bhatia S., Bera T. Classical and nonclassical techniques for secondary metabolite production in plant cell culture. In: Bhatia S., Sharma K., Dahiya R., Bera T., editors. Modern Applications of Plant Biotechnology in Pharmaceutical Sciences. Elsevier; Amsterdam, The Netherlands: 2015. pp. 231–291.
LinkOut - more resources
Full Text Sources
Research Materials

