In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum lucentifolium Cronquist
- PMID: 35009097
- PMCID: PMC8747116
- DOI: 10.3390/plants11010094
In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum lucentifolium Cronquist
Abstract
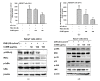
UVB exposure causes DNA mutation and ROS generation, which lead to skin photoaging, skin wrinkling, skin sagging, and uneven skin pigmentation. ROS activate the NF-κB and MAPK signaling pathways leading to production of inflammatory molecules such as COX-2, collagen-degrading proteins such as matrix metalloproteinases (MMPs), and moisture-deficiency-related proteins such as hyaluronidases (HYALs). UVB exposure also induces irregular skin pigmentation though melanin overproduction, related to CREB transcription factor activity and transcription of melanogenesis genes. Here, we demonstrate that Chrysophyllum lucentifolium methanol extract (Cl-ME) has antioxidant activity; it dose-dependently decreased the expression of COX-2, MMP-1, MMP-9, HYAL-1, and HYAL-4 by downregulating the NF-κB (IKKα/β, IκBα) and MAPK (ERK, JNK, and p38) pathways and increased the expression of Col1a1, which encodes a protein important for maintaining skin elasticity. Cl-ME also showed promising antimelanogenic activity by decreasing the expression of CREB, a transcription factor, which in turn inhibited the expression of genes encoding tyrosinase, MITF, TYRP1, and TYRP2. In summary, a methanol extract of C. lucentifolium exhibited antiphotoaging and antimelanogenic activity and could be useful in the cosmeceutical industry.
Keywords: AP-1; NF-κB; ROS; skin aging; skin whitening.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

