Dual Thermo- and Photo-Responsive Micelles Based on Azobenzene-Containing Random Copolymer
- PMID: 35009149
- PMCID: PMC8746059
- DOI: 10.3390/ma15010002
Dual Thermo- and Photo-Responsive Micelles Based on Azobenzene-Containing Random Copolymer
Abstract
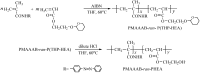
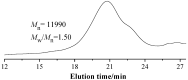
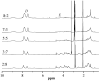
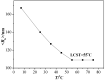
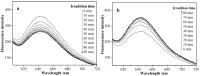
Amphiphilic random copolymer poly(methacrylamido-azobenzene)-ran-poly(2-hydroxyethylacrylate) (PMAAAB-ran-PHEA) was synthesized via hydrolysis of poly(methacrylamido-azobenzene)-ran-poly[2-((2'-tetrahydropyranyl)oxy)ethylacrylate] (PMAAAB-ran-P(THP-HEA)), which was prepared by conventional radical polymerization. PMAAAB-ran-PHEA micelles were then prepared via dialysis method against water with DMF as solvent. The structure, morphology, size, and low critical solution temperature (LCST) of PMAAAB-ran-PHEA and its micelles were determined by 1H-NMR, GPC, TEM, and DLS. The thermo- and photo-responsive behaviors of the resulting polymer micelles were investigated with Nile red as a fluorescence probe. The results showed that PMAAAB-ran-PHEA micelles were porous or bowl-shaped and its size was 135-150 nm, and its LCST was 55 °C when FMAAAB of the random copolymer was 0.5351; the hydrophobicity of the micellar core was changed reversibly under the irradiation of UV light and visible light without release of Nile red or disruption of micelles; the size and solubilization capacity of the micelles were dependent on temperature, and Nile red would migrate for many times between the water phase and the micelles, and finally increasingly accumulated during the repeated heating and cooling processes.
Keywords: 2-hydroxyethylacrylate; Nile red; amphiphilic random copolymer; methacrylamido-azobenzene; micelles; photo-/thermo-responsiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Wei M., Gao Y., Li X., Serpe M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017;8:127–143. doi: 10.1039/C6PY01585A. - DOI
Grants and funding
- 2014GXNSFAA118042/General project of Guangxi Natural Science Foundation
- 206Z1402G/Local science and technology development fund projects guided by the central government
- ZD2018311,ZD2020417/Key projects of science and technology research in Hebei higher education institution
- 2019ZC006, 2019ZC049,2019ZZ023, 2020ZZ040,2019ZX07/Xingtai science and technology program
- 21064001/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

