Preliminary Characterization of a Polycaprolactone-SurgihoneyRO Electrospun Mesh for Skin Tissue Engineering
- PMID: 35009233
- PMCID: PMC8746156
- DOI: 10.3390/ma15010089
Preliminary Characterization of a Polycaprolactone-SurgihoneyRO Electrospun Mesh for Skin Tissue Engineering
Abstract
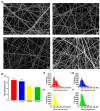
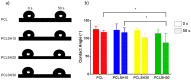
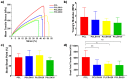
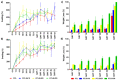
Skin is a hierarchical and multi-cellular organ exposed to the external environment with a key protective and regulatory role. Wounds caused by disease and trauma can lead to a loss of function, which can be debilitating and even cause death. Accelerating the natural skin healing process and minimizing the risk of infection is a clinical challenge. Electrospinning is a key technology in the development of wound dressings and skin substitutes as it enables extracellular matrix-mimicking fibrous structures and delivery of bioactive materials. Honey is a promising biomaterial for use in skin tissue engineering applications and has antimicrobial properties and potential tissue regenerative properties. This preliminary study investigates a solution electrospun composite nanofibrous mesh based on polycaprolactone and a medical grade honey, SurgihoneyRO. The processing conditions were optimized and assessed by scanning electron microscopy to fabricate meshes with uniform fiber diameters and minimal presence of beads. The chemistry of the composite meshes was examined using Fourier transform infrared spectroscopy and X-ray photon spectroscopy showing incorporation of honey into the polymer matrix. Meshes incorporating honey had lower mechanical properties due to lower polymer content but were more hydrophilic, resulting in an increase in swelling and an accelerated degradation profile. The biocompatibility of the meshes was assessed using human dermal fibroblasts and adipose-derived stem cells, which showed comparable or higher cell metabolic activity and viability for SurgihoneyRO-containing meshes compared to polycaprolactone only meshes. The meshes showed no antibacterial properties in a disk diffusion test due to a lack of hydrogen peroxide production and release. The developed polycaprolactone-honey nanofibrous meshes have potential for use in skin applications.
Keywords: electrospinning; honey; polycaprolactone; skin.
Conflict of interest statement
C.V., J.Y.M., G.H., C.D., and P.B. are currently involved in a separate project with Matoke Holding.
Figures




References
-
- Timmons J. Skin function and wound healing physiology. Wound Essent. 2006;1:8–17.
-
- Dias J.R., Granja P.L., Bártolo P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016;84:314–334. doi: 10.1016/j.pmatsci.2016.09.006. - DOI
-
- Velnar T., Bailey T., Smrkolj V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. SAGE; Los Angeles, CA, USA: 2009. pp. 1528–1542. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

