Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles
- PMID: 35009367
- PMCID: PMC8746074
- DOI: 10.3390/ma15010213
Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles
Abstract
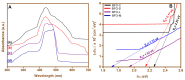
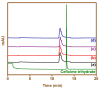
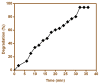
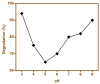
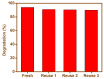
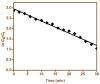
The present work was carried out to synthesize bismuth ferrite (BFO) nanoparticles by combustion synthesis, and to evaluate the photocatalytic activity of synthesized bismuth ferrite nanoparticles against cefixime trihydrate. BFO nanoparticles were successfully synthesized using bismuth (III) nitrate and iron (III) nitrate by a combustion synthesis method employing different types of fuels such as maltose, succinic acid, cinnamic acid, and lactose. The effects of the different types of fuels on the morphology and size of the bismuth ferrite nanoparticles were investigated. Characterization of the as-obtained bismuth ferrite nanoparticles was carried out by different techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), Energy-Dispersive Spectroscopy (EDS), N2-sorption analysis, Fourier-transform infrared spectroscopy (FT-IR), and ultraviolet-visible (UV-vis) spectroscopy. Photoluminescence studies were also carried out for the various bismuth ferrite nanoparticles obtained. Degradation of cefixime trihydrate was investigated under sunlight to evaluate the photocatalytic properties of the bismuth ferrite nanoparticles, and it was found that the bismuth ferrite nanoparticles followed first-order degradation kinetics in solar irradiation in the degradation of antibiotic, cefixime trihydrate.
Keywords: BiFeO3; cephalosporin; photocatalysis; sunlight.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Mahmood A.R., Al-Haideri H.H., Hassan F.M. Detection of Antibiotics in Drinking Water Treatment Plants in Baghdad City, Iraq. Adv. Public Health. 2019;2019:7851354. doi: 10.1155/2019/7851354. - DOI
-
- Storz G., Hengge R. Bacterial Stress Responses. American Society for Microbiology Press; Washington, DC, USA: 2010.
-
- Glassmeyer S.T., Hinchey E.K., Boehme S.E., Daughton C.G., Ruhoy I.S., Conerly O., Daniels R.L., Lauer L., McCarthy M., Nettesheim T.G., et al. Disposal practices for unwanted residential medications in the United States. Environ. Int. 2009;35:566–572. doi: 10.1016/j.envint.2008.10.007. - DOI - PubMed
-
- Hussain S., Naeem M., Chaudhry M.N. Estimation of Residual Antibiotics in Pharmaceutical Effluents and their Fate in Affected Areas. Pol. J. Environ. Stud. 2016;25:607–614. doi: 10.15244/pjoes/61229. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

