Upconversion Nanoparticles-Based Fluorescence Immunoassay for the Sensitive Detection of 2-Amino-3-methylimidazo [4,5-f] Quinoline (IQ) in Heat Processed Meat
- PMID: 35009550
- PMCID: PMC8747629
- DOI: 10.3390/s22010008
Upconversion Nanoparticles-Based Fluorescence Immunoassay for the Sensitive Detection of 2-Amino-3-methylimidazo [4,5-f] Quinoline (IQ) in Heat Processed Meat
Abstract
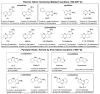
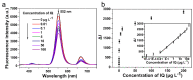
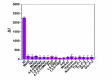
A competitive fluorescence immunoassay for the quantitative detection of 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) in pan-fried meat patties was developed, using magnetic nanoparticles coupled with coating antigen as the capture probe and anti-IQ antibody coupled with NaYF4: Yb, Er upconversion nanoparticles as the signal probe. Under optimal conditionals, the wide detection range for IQ in phosphate buffer saline is from 0.01 to 100 μg·L-1 (R2 = 0.991) with a detection limit of 0.007 μg·L-1. This proposed method has been applied to detect IQ in two different types of pan-fried meat patties at varying frying times, and the IQ content in chicken patties and fish patties are 2.11-3.47 μg·kg-1 and 1.35-2.85 μg·kg-1, respectively. These results are consistent with that of the ultraperformance liquid chromatography-tandem mass spectrometry. In summary, this method can serve as a sensitive and specific test tool for the determination of IQ in processed meat.
Keywords: 2-amino-3-methylimidazo [4,5-f] quinoline; fluorescence immunoassay; heat processed meat; magnetic separation; upconversion nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jägerstad M., Skog K., Arvidsson P., Solyakov A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Z. Lebensm.-Forsch. A. 1998;207:419–427. doi: 10.1007/s002170050355. - DOI
-
- Ohgaki H., Kusama K., Matsukura N., Morino K., Hasegawa H., Sato S., Takayama S., Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3-methylimidazo [4,5-f] quinoline, from broiled sardine, cooked beef and beef extract. Carcinogenesis. 1984;5:921–924. doi: 10.1093/carcin/5.7.921. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

