Early Diagnosis of Multiple Sclerosis Using Swept-Source Optical Coherence Tomography and Convolutional Neural Networks Trained with Data Augmentation
- PMID: 35009710
- PMCID: PMC8747672
- DOI: 10.3390/s22010167
Early Diagnosis of Multiple Sclerosis Using Swept-Source Optical Coherence Tomography and Convolutional Neural Networks Trained with Data Augmentation
Abstract
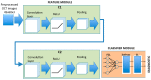
Background: The aim of this paper is to implement a system to facilitate the diagnosis of multiple sclerosis (MS) in its initial stages. It does so using a convolutional neural network (CNN) to classify images captured with swept-source optical coherence tomography (SS-OCT).
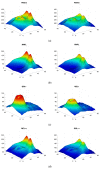
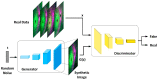
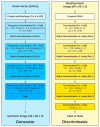
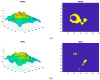
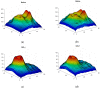
Methods: SS-OCT images from 48 control subjects and 48 recently diagnosed MS patients have been used. These images show the thicknesses (45 × 60 points) of the following structures: complete retina, retinal nerve fiber layer, two ganglion cell layers (GCL+, GCL++) and choroid. The Cohen distance is used to identify the structures and the regions within them with greatest discriminant capacity. The original database of OCT images is augmented by a deep convolutional generative adversarial network to expand the CNN's training set.
Results: The retinal structures with greatest discriminant capacity are the GCL++ (44.99% of image points), complete retina (26.71%) and GCL+ (22.93%). Thresholding these images and using them as inputs to a CNN comprising two convolution modules and one classification module obtains sensitivity = specificity = 1.0.
Conclusions: Feature pre-selection and the use of a convolutional neural network may be a promising, nonharmful, low-cost, easy-to-perform and effective means of assisting the early diagnosis of MS based on SS-OCT thickness data.
Keywords: convolutional neural network; generative adversarial network; multiple sclerosis; optical coherence tomography.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Ker J., Wang L., Rao J., Lim T. Deep Learning Applications in Medical Image Analysis. IEEE Access. 2018;6:9375–9389. doi: 10.1109/ACCESS.2017.2788044. - DOI
-
- Salem M., Valverde S., Cabezas M., Pareto D., Oliver A., Salvi J., Rovira A., Llado X. Multiple Sclerosis Lesion Synthesis in MRI Using an Encoder-Decoder U-NET. IEEE Access. 2019;7:25171–25184. doi: 10.1109/ACCESS.2019.2900198. - DOI
-
- McKinley R., Wepfer R., Grunder L., Aschwanden F., Fischer T., Friedli C., Muri R., Rummel C., Verma R., Weisstanner C., et al. Automatic detection of lesion load change in Multiple Sclerosis using convolutional neural networks with segmentation confidence. NeuroImage Clin. 2020;25:102104. doi: 10.1016/j.nicl.2019.102104. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

