Carbon Dioxide Sensing-Biomedical Applications to Human Subjects
- PMID: 35009731
- PMCID: PMC8749784
- DOI: 10.3390/s22010188
Carbon Dioxide Sensing-Biomedical Applications to Human Subjects
Abstract
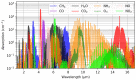
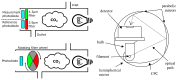
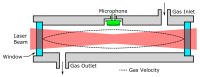
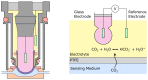
Carbon dioxide (CO2) monitoring in human subjects is of crucial importance in medical practice. Transcutaneous monitors based on the Stow-Severinghaus electrode make a good alternative to the painful and risky arterial "blood gases" sampling. Yet, such monitors are not only expensive, but also bulky and continuously drifting, requiring frequent recalibrations by trained medical staff. Aiming at finding alternatives, the full panel of CO2 measurement techniques is thoroughly reviewed. The physicochemical working principle of each sensing technique is given, as well as some typical merit criteria, advantages, and drawbacks. An overview of the main CO2 monitoring methods and sites routinely used in clinical practice is also provided, revealing their constraints and specificities. The reviewed CO2 sensing techniques are then evaluated in view of the latter clinical constraints and transcutaneous sensing coupled to a dye-based fluorescence CO2 sensing seems to offer the best potential for the development of a future non-invasive clinical CO2 monitor.
Keywords: CO2; carbon dioxide; paCO2; ptCO2; tcpCO2; transcutaneous monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

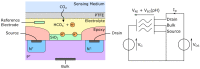
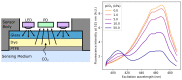
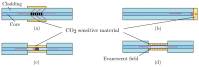

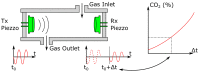
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

