Functionalized Nanomaterials as Tailored Theranostic Agents in Brain Imaging
- PMID: 35009968
- PMCID: PMC8746658
- DOI: 10.3390/nano12010018
Functionalized Nanomaterials as Tailored Theranostic Agents in Brain Imaging
Abstract
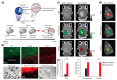
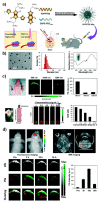
Functionalized nanomaterials of various categories are essential for developing cancer nano-theranostics for brain diseases; however, some limitations exist in their effectiveness and clinical translation, such as toxicity, limited tumor penetration, and inability to cross blood-brain and blood-tumor barriers. Metal nanomaterials with functional fluorescent tags possess unique properties in improving their functional properties, including surface plasmon resonance (SPR), superparamagnetism, and photo/bioluminescence, which facilitates imaging applications in addition to their deliveries. Moreover, these multifunctional nanomaterials could be synthesized through various chemical modifications on their physical surfaces via attaching targeting peptides, fluorophores, and quantum dots (QD), which could improve the application of these nanomaterials by facilitating theranostic modalities. In addition to their inherent CT (Computed Tomography), MRI (Magnetic Resonance Imaging), PAI (Photo-acoustic imaging), and X-ray contrast imaging, various multifunctional nanoparticles with imaging probes serve as brain-targeted imaging candidates in several imaging modalities. The primary criteria of these functional nanomaterials for translational application to the brain must be zero toxicity. Moreover, the beneficial aspects of nano-theranostics of nanoparticles are their multifunctional systems proportioned towards personalized disease management via comprising diagnostic and therapeutic abilities in a single biodegradable nanomaterial. This review highlights the emerging aspects of engineered nanomaterials to reach and deliver therapeutics to the brain and how to improve this by adopting the imaging modalities for theranostic applications.
Keywords: contrast agents; delivery; functionalized nanomaterials; imaging; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Polymeric multifunctional nanomaterials for theranostics.J Mater Chem B. 2015 Sep 14;3(34):6856-6870. doi: 10.1039/c5tb00617a. Epub 2015 Jul 9. J Mater Chem B. 2015. PMID: 32262535
-
Nanoparticles in practice for molecular-imaging applications: An overview.Acta Biomater. 2016 Sep 1;41:1-16. doi: 10.1016/j.actbio.2016.06.003. Epub 2016 Jun 2. Acta Biomater. 2016. PMID: 27265153 Review.
-
Advancements in Applications of Surface Modified Nanomaterials for Cancer Theranostics.Curr Drug Metab. 2017;18(11):983-999. doi: 10.2174/1389200218666171002122039. Curr Drug Metab. 2017. PMID: 28969548 Review.
-
Theranostic Applications of Nanomaterials in Alzheimer's Disease: A Multifunctional Approach.Curr Pharm Des. 2022;28(2):116-132. doi: 10.2174/1381612827666211122153946. Curr Pharm Des. 2022. PMID: 34809540 Review.
-
Nanomaterial Probes for Nuclear Imaging.Nanomaterials (Basel). 2022 Feb 9;12(4):582. doi: 10.3390/nano12040582. Nanomaterials (Basel). 2022. PMID: 35214911 Free PMC article. Review.
Cited by
-
Cancer theragnostics: closing the loop for advanced personalized cancer treatment through the platform integration of therapeutics and diagnostics.Front Bioeng Biotechnol. 2025 Jan 17;12:1499474. doi: 10.3389/fbioe.2024.1499474. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39898278 Free PMC article. Review.
-
Magnetic nanoparticles for ferroptosis cancer therapy with diagnostic imaging.Bioact Mater. 2023 Sep 29;32:66-97. doi: 10.1016/j.bioactmat.2023.09.015. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 37822917 Free PMC article. Review.
-
Peptides to Overcome the Limitations of Current Anticancer and Antimicrobial Nanotherapies.Pharmaceutics. 2022 Jun 10;14(6):1235. doi: 10.3390/pharmaceutics14061235. Pharmaceutics. 2022. PMID: 35745807 Free PMC article. Review.
-
The Potential of Novel Synthesized Carbon Dots Derived Resveratrol Using One-Pot Green Method in Accelerating in vivo Wound Healing.Int J Nanomedicine. 2023 Nov 18;18:6813-6828. doi: 10.2147/IJN.S434071. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38026533 Free PMC article.
-
Scope and challenges of nanoparticle-based mRNA delivery in cancer treatment.Arch Pharm Res. 2022 Dec;45(12):865-893. doi: 10.1007/s12272-022-01418-x. Epub 2022 Nov 24. Arch Pharm Res. 2022. PMID: 36422795 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

