Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection
- PMID: 35009984
- PMCID: PMC8746978
- DOI: 10.3390/nano12010033
Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection
Abstract
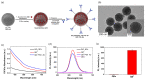
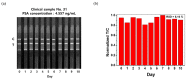
Prostate cancer can be detected early by testing the presence of prostate-specific antigen (PSA) in the blood. Lateral flow immunoassay (LFIA) has been used because it is cost effective and easy to use and also has a rapid sample-to-answer process. Quantum dots (QDs) with very bright fluorescence have been previously used to improve the detection sensitivity of LFIAs. In the current study, a highly sensitive LFIA kit was devised using QD-embedded silica nanoparticles. In the present study, only a smartphone and a computer software program, ImageJ, were used, because the developed system had high sensitivity by using very bright nanoprobes. The limit of PSA detection of the developed LFIA system was 0.138 ng/mL. The area under the curve of this system was calculated as 0.852. The system did not show any false-negative result when 47 human serum samples were analyzed; it only detected PSA and did not detect alpha-fetoprotein and newborn calf serum in the samples. Additionally, fluorescence was maintained on the strip for 10 d after the test. With its high sensitivity and convenience, the devised LFIA kit can be used for the diagnosis of prostate cancer.
Keywords: lateral flow immunoassay; prostate cancer; prostate-specific antigen; quantum dot; quantum-dot-embedded silica nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

