MOFs-Derived Zn-Based Catalysts in Acetylene Acetoxylation
- PMID: 35010047
- PMCID: PMC8746958
- DOI: 10.3390/nano12010098
MOFs-Derived Zn-Based Catalysts in Acetylene Acetoxylation
Abstract
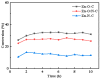
Metal-organic frameworks (MOFs)-derived materials with a large specific surface area and rich pore structures are favorable for catalytic performance. In this work, MOFs are successfully prepared. Through pyrolysis of MOFs under nitrogen gas, zinc-based catalysts with different active sites for acetylene acetoxylation are obtained. The influence of the oxygen atom, nitrogen atom, and coexistence of oxygen and nitrogen atoms on the structure and catalytic performance of MOFs-derived catalysts was investigated. According to the results, the catalysts with different catalytic activity are Zn-O-C (33%), Zn-O/N-C (27%), and Zn-N-C (12%). From the measurements of X-ray photoelectron spectroscopy (XPS), it can be confirmed that the formation of different active sites affects the electron cloud density of zinc. The electron cloud density of zinc affects the ability to attract CH3COOH, which makes catalysts different in terms of catalytic activity.
Keywords: acetylene acetoxylation; heteroatoms; metal–organic frameworks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ping L.I., Feng L.R., Jian L.Z., You K.S., Yuan M.J., Qiu F.L. A Research Summary of Vinyl Acetate Synthesis from Acetic Acid and Acetylene Catalyzed by Active Carbon-zinc Acetate. Nat. Sci. J. Hainan Univ. 2006;9:15886. doi: 10.15886/j.cnki.hdxbzkb.2006.04.009. - DOI
-
- Zhang M., Zhuang J., Wu X., Yu Y. Experimental and theoretical insights into the cyclotrimerization of acetylene during vinyl acetate synthesis. Chem. Eng. J. 2019;378:122183. doi: 10.1016/j.cej.2019.122183. - DOI
-
- Dong X., Wang Y., Yu Y., Zhang M. Density Functional Theory Investigation on the Synthesis Mechanism of Vinyl Acetate from Acetylene and Acetic Acid Catalyzed by Ordered Mesoporous Carbon-Supported Zinc Acetate. Ind. Eng. Chem. Res. 2018;57:7363–7373. doi: 10.1021/acs.iecr.8b00596. - DOI
-
- Xing B., Wei Z., Wang G. Acetate coverage effect on the reactivity of vinyl acetate synthesis on Pd/Au alloy surfaces. J. Energy Chem. 2013;671:600893. doi: 10.1016/S2095-4956(13)60089-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

