The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process
- PMID: 35010673
- PMCID: PMC8745009
- DOI: 10.3390/ijerph19010413
The Importance of Direct Patient Reporting of Adverse Drug Reactions in the Safety Monitoring Process
Abstract
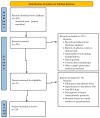
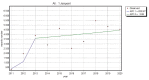
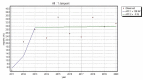
All medicinal products authorized in the European Union are subjects of constant drug-safety monitoring processes. It is organized in a pharmacovigilance system that is designed to protect human health and life by the detection, analysis and prevention of adverse drug reactions (ADRs) and other drug-related problems. The main role of the aforementioned system is to collect and analyze adverse drug reaction reports. Legislation introduced several years ago allowed patients, their legal representatives and caregivers to report adverse drug reactions, which caused them to be an additional source of safety data. This paper presents the analysis of EudraVigilance data related to adverse drug reactions provided by patients, their representatives, as well as those obtained from healthcare professionals related to medicines which belong to M01A anti-inflammatory and antirheumatic products, a non-steroid group. The objective of the study was to identify the changes in the number and structure of adverse reaction reporting after the introduction of pharmacovigilance (PV) obligations in EU. A review of scientific literature was also conducted to assess the differences in adverse reactions reported by patients or their representatives and by healthcare professionals. We also identified other factors which, according to literature review, influenced the number of adverse reaction reports provided by patients. Analysis of data collected from the EudraVigilance showed that from 2011 to 2013 the number of reports made by patients and their caregivers increased by approx. 24 percentage points, and then, from 2014, it constituted around 30% of the total of reported reactions every year, so patient reporting is an important part of pharmacovigilance system and a source of drugs' safety information throughout their use in healthcare practice. Additionally, there was no interrelationship between the seriousness of reported adverse reactions and the overall number of patient reports when compared to reports form healthcare professionals.
Keywords: EudraVigilance; adverse reaction; patient reporting; pharmacovigilance; safety monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pharmacovigilance: Overview. [(accessed on 30 October 2021)]. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/pharmacovigilance....
-
- World Health Organization . The Importance of Pharmacovigilance. World Health Organization; Geneva, Switzerland: 2002. Pharmacovigilance in drug regulation; pp. 15–17.
-
- Commission Implementing Regulation (EU) No 520/2012 of 19 June 2012 on the Performance of Pharmacovigilance Activities Provided for in Regulation (EC) No 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council L 159/5 20/6/2012. [(accessed on 16 November 2021)]. Available online: https://eur-lex.europa.eu/eli/reg_impl/2012/520/oj.
-
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. [(accessed on 16 January 2021)]. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32001L0083.
-
- Guideline on Good Pharmacovigilance Practices (GVP) Module I—Pharmacovigilance Systems and Their Quality Systems 22 June 2012 EMA/541760/2011. [(accessed on 30 October 2021)]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-go....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

