An Investigation into the Effects of a Curcumin Extract (Curcugen®) on Osteoarthritis Pain of the Knee: A Randomised, Double-Blind, Placebo-Controlled Study
- PMID: 35010916
- PMCID: PMC8746505
- DOI: 10.3390/nu14010041
An Investigation into the Effects of a Curcumin Extract (Curcugen®) on Osteoarthritis Pain of the Knee: A Randomised, Double-Blind, Placebo-Controlled Study
Abstract
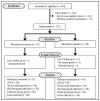
Curcumin, a phytochemical from the spice turmeric, has anti-inflammatory properties and has been shown to have pain-relieving effects. In this 8-week, randomised, double-blind, placebo-controlled study, 101 adults with knee osteoarthritis received either 500 mg twice daily of a standardised curcumin extract (Curcugen®) or placebo. Outcome measures included the Knee Injury and Osteoarthritis Outcome Score (KOOS), knee pain ratings, Japanese Orthopaedic Association Score for Osteoarthritic Knees (JOA), PROMIS-29, and performance-based testing comprising the 40-m fast-paced walk test, 6-min walk test, timed up-and-go test, and 30-s chair stand test. Compared to the placebo, curcumin significantly reduced the KOOS knee pain score (p = 0.009) and numeric knee pain ratings (p = 0.001). Curcumin was also associated with greater improvements (p ≤ 0.05) than the placebo on the timed up-and-go test, 6-min walk test, and the JOA total score; but not the 30-s chair stand test or 40-m fast-paced walk test. Pain-relieving medication was reduced in 37% of participants on curcumin compared to 13% on placebo. The findings support the potential efficacy of curcumin for the treatment of osteoarthritis of the knee but studies of longer duration, varying treatment doses, differing curcumin extracts, and the use of other objective outcome measures will be helpful to expand on these findings.
Keywords: clinical trial; curcumin; knee; osteoarthritis; turmeric.
Conflict of interest statement
A.L.L. is the managing director of Clinical Research Australia, a contract research organisation that has received research funding from nutraceutical companies. A.L.L. has also received presentation honoraria from nutraceutical companies. S.J.S. is an employee of Clinical Research Australia and declares no other conflicts of interest. T.F. declares no conflicts of interest. S.J.-M. is the director of medical & scientific affairs at Dolcas-Biotech LLC. Dolcas Biotech was involved in the study design and writing of the manuscript but was not involved in data collection or analysis and interpretation of data.
Figures

References
-
- Feigin V., GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

