Lactoferrin Prevents Hepatic Injury and Fibrosis via the Inhibition of NF-κB Signaling in a Rat Non-Alcoholic Steatohepatitis Model
- PMID: 35010924
- PMCID: PMC8746867
- DOI: 10.3390/nu14010042
Lactoferrin Prevents Hepatic Injury and Fibrosis via the Inhibition of NF-κB Signaling in a Rat Non-Alcoholic Steatohepatitis Model
Abstract
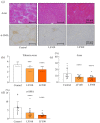
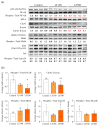
Non-alcoholic steatohepatitis (NASH) can cause liver cirrhosis and hepatocellular carcinoma (HCC), with cases increasing worldwide. To reduce the incidence of liver cirrhosis and HCC, NASH is targeted for the development of treatments, along with viral hepatitis and alcoholic hepatitis. Lactoferrin (LF) has antioxidant, anti-cancer, and anti-inflammatory activities. However, whether LF affects NASH and fibrosis remains unelucidated. We aimed to clarify the chemopreventive effect of LF on NASH progression. We used a NASH model with metabolic syndrome established using connexin 32 (Cx32) dominant negative transgenic (Cx32ΔTg) rats. Cx32ΔTg rats (7 weeks old) were fed a high-fat diet and intraperitoneally injected with dimethylnitrosamine (DMN). Rats were divided into three groups for LF treatment at 0, 100, or 500 mg/kg/day for 17 weeks. Lactoferrin significantly protected steatosis and lobular inflammation in Cx32ΔTg rat livers and attenuated bridging fibrosis or liver cirrhosis induced by DMN. By quantitative RT-PCR, LF significantly down-regulated inflammatory (Tnf-α, Il-6, Il-18, and Il-1β) and fibrosis-related (Tgf-β1, Timp2, and Col1a1) cytokine mRNAs. Phosphorylated nuclear factor (NF)-κB protein decreased in response to LF, while phosphorylated JNK protein was unaffected. These results indicate that LF might act as a chemopreventive agent to prevent hepatic injury, inflammation, and fibrosis in NASH via NF-κB inactivation.
Keywords: NASH; connexin; fibrosis; hepatocarcinogenesis; lactoferrin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

