Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome-Host Interaction Mechanistic Hypothesis
- PMID: 35010940
- PMCID: PMC8746684
- DOI: 10.3390/nu14010065
Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome-Host Interaction Mechanistic Hypothesis
Abstract
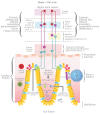
Dysbiosis secondary to environmental factors, including dietary patterns, antibiotics use, pollution exposure, and other lifestyle factors, has been associated to many non-infective chronic inflammatory diseases. Autism spectrum disorder (ASD) is related to maternal inflammation, although there is no conclusive evidence that affected individuals suffer from systemic low-grade inflammation as in many psychological and psychiatric diseases. However, neuro-inflammation and neuro-immune abnormalities are observed within ASD-affected individuals. Rebalancing human gut microbiota to treat disease has been widely investigated with inconclusive and contradictory findings. These observations strongly suggest that the forms of dysbiosis encountered in ASD-affected individuals could also originate from autonomic nervous system (ANS) functioning abnormalities, a common neuro-anatomical alteration underlying ASD. According to this hypothesis, overactivation of the sympathetic branch of the ANS, due to the fact of an ASD-specific parasympathetic activity deficit, induces deregulation of the gut-brain axis, attenuating intestinal immune and osmotic homeostasis. This sets-up a dysbiotic state, that gives rise to immune and osmotic dysregulation, maintaining dysbiosis in a vicious cycle. Here, we explore the mechanisms whereby ANS imbalances could lead to alterations in intestinal microbiome-host interactions that may contribute to the severity of ASD by maintaining the brain-gut axis pathways in a dysregulated state.
Keywords: autism spectrum disorder (ASD); autonomic nervous system (ANS); brain–gut axis (BGA); dysbiosis; gastrointestinal (GI) tract; microbiome; neurodevelopment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E., Gehn E., Loresto M., Mitchell J., Atwood S., et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011;8:34. doi: 10.1186/1743-7075-8-34. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

