Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance
- PMID: 35010945
- PMCID: PMC8746507
- DOI: 10.3390/nu14010067
Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance
Abstract
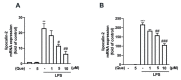
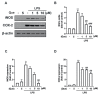
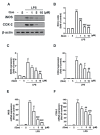
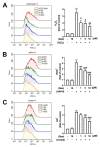
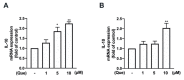
Macrophage polarization plays essential and diverse roles in most diseases, such as atherosclerosis, adipose tissue inflammation, and insulin resistance. Homeostasis dysfunction in M1/M2 macrophage polarization causes pathological conditions and inflammation. Neuroinflammation is characterized by microglial activation and the concomitant production of pro-inflammatory cytokines, leading to numerous neurodegenerative diseases and psychiatric disorders. Decreased neuroinflammation can be obtained by using natural compounds, including flavonoids, which are known to ameliorate inflammatory responses. Among flavonoids, quercetin possesses multiple pharmacological applications and regulates several biological activities. In the present study, we found that quercetin effectively inhibited the expression of lipocalin-2 in both macrophages and microglial cells stimulated by lipopolysaccharides (LPS). The production of nitric oxide (NO) and expression levels of the pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2, were also attenuated by quercetin treatment. Our results also showed that quercetin significantly reduced the expression levels of the M1 markers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β, in the macrophages and microglia. The M1 polarization-associated chemokines, C-C motif chemokine ligand (CCL)-2 and C-X-C motif chemokine ligand (CXCL)-10, were also effectively reduced by the quercetin treatment. In addition, quercetin markedly reduced the production of various reactive oxygen species (ROS) in the microglia. The microglial phagocytic ability induced by the LPS was also effectively reduced by the quercetin treatment. Importantly, the quercetin increased the expression levels of the M2 marker, IL-10, and the endogenous antioxidants, heme oxygenase (HO)-1, glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), and NAD(P)H quinone oxidoreductase-1 (NQO1). The enhancement of the M2 markers and endogenous antioxidants by quercetin was activated by the AMP-activated protein kinase (AMPK) and Akt signaling pathways. Together, our study reported that the quercetin inhibited the effects of M1 polarization, including neuroinflammatory responses, ROS production, and phagocytosis. Moreover, the quercetin enhanced the M2 macrophage polarization and endogenous antioxidant expression in both macrophages and microglia. Our findings provide valuable information that quercetin may act as a potential drug for the treatment of diseases related to inflammatory disorders in the central nervous system.
Keywords: homeostasis; inflammation; macrophage; microglia; oxidative stress; quercetin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Song M.G., Ryoo I.G., Choi H.Y., Choi B.H., Kim S.T., Heo T.H., Lee J.Y., Park P.H., Kwak M.K. NRF2 Signaling Negatively Regulates Phorbol-12-Myristate-13-Acetate (PMA)-Induced Differentiation of Human Monocytic U937 Cells into Pro-Inflammatory Macrophages. PLoS ONE. 2015;10:e0134235. doi: 10.1371/journal.pone.0134235. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

