Oxidative Crosslinking of Peptides and Proteins: Mechanisms of Formation, Detection, Characterization and Quantification
- PMID: 35011250
- PMCID: PMC8746199
- DOI: 10.3390/molecules27010015
Oxidative Crosslinking of Peptides and Proteins: Mechanisms of Formation, Detection, Characterization and Quantification
Abstract
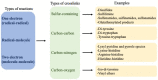
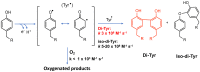
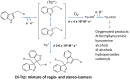
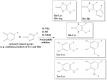
Covalent crosslinks within or between proteins play a key role in determining the structure and function of proteins. Some of these are formed intentionally by either enzymatic or molecular reactions and are critical to normal physiological function. Others are generated as a consequence of exposure to oxidants (radicals, excited states or two-electron species) and other endogenous or external stimuli, or as a result of the actions of a number of enzymes (e.g., oxidases and peroxidases). Increasing evidence indicates that the accumulation of unwanted crosslinks, as is seen in ageing and multiple pathologies, has adverse effects on biological function. In this article, we review the spectrum of crosslinks, both reducible and non-reducible, currently known to be formed on proteins; the mechanisms of their formation; and experimental approaches to the detection, identification and characterization of these species.
Keywords: aggregation; crosslink; di-tryptophan; di-tyrosine; dimerization; disulfides; mass spectrometry; protein oxidation; proteomics; radicals; thiols.
Conflict of interest statement
M.J.D. declares consultancy contracts with Novo Nordisk A/S. This funder had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

