Constrained Dipeptide Surrogates: 5- and 7-Hydroxy Indolizidin-2-one Amino Acid Synthesis from Iodolactonization of Dehydro-2,8-diamino Azelates
- PMID: 35011297
- PMCID: PMC8746717
- DOI: 10.3390/molecules27010067
Constrained Dipeptide Surrogates: 5- and 7-Hydroxy Indolizidin-2-one Amino Acid Synthesis from Iodolactonization of Dehydro-2,8-diamino Azelates
Abstract
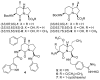
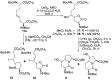
The constrained dipeptide surrogates 5- and 7-hydroxy indolizidin-2-one N-(Boc)amino acids have been synthesized from L-serine as a chiral educt. A linear precursor ∆4-unsaturated (2S,8S)-2,8-bis[N-(Boc)amino]azelic acid was prepared in five steps from L-serine. Although epoxidation and dihydroxylation pathways gave mixtures of hydroxy indolizidin-2-one diastereomers, iodolactonization of the ∆4-azelate stereoselectively delivered a lactone iodide from which separable (5S)- and (7S)-hydroxy indolizidin-2-one N-(Boc)amino esters were synthesized by sequences featuring intramolecular iodide displacement and lactam formation. X-ray analysis of the (7S)-hydroxy indolizidin-2-one N-(Boc)amino ester indicated that the backbone dihedral angles embedded in the bicyclic ring system resembled those of the central residues of an ideal type II' β-turn indicating the potential for peptide mimicry.
Keywords: heterocycle; indolizidin-2-one amino acids; iodolactonization; lactam; peptide mimic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hanessian S., McNaughton-Smith G., Lombart H.G., Lubell W.D. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron. 1997;53:12789–12854. doi: 10.1016/S0040-4020(97)00476-6. - DOI
-
- Beausoleil E., Lubell W.D. Steric Effects on the Amide Isomer Equilibrium of Prolyl Peptides. Synthesis and Conformational Analysis of N-Acetyl-5-tert-butylproline N′-Methylamides. J. Am. Chem. Soc. 1996;118:12902–12908. doi: 10.1021/ja962013b. - DOI
-
- Hamdane Y., Chauhan P.S., Vutla S., Mulumba M., Ong H., Lubell W.D. 5-Substituted N-Aminoimidazolone Peptide Mimic Synthesis by Organocatalyzed Reactions of Azopeptides and Use in the Analysis of Biologically Active Backbone and Side-Chain Topology. Org. Lett. 2021;23:3491–3495. doi: 10.1021/acs.orglett.1c00936. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

