Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands
- PMID: 35011327
- PMCID: PMC8746573
- DOI: 10.3390/molecules27010095
Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands
Abstract
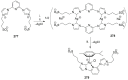
Since the discovery of persistent carbenes by the isolation of 1,3-di-l-adamantylimidazol-2-ylidene by Arduengo and coworkers, we witnessed a fast growth in the design and applications of this class of ligands and their metal complexes. Modular synthesis and ease of electronic and steric adjustability made this class of sigma donors highly popular among chemists. While the nature of the metal-carbon bond in transition metal complexes bearing N-heterocyclic carbenes (NHCs) is predominantly considered to be neutral sigma or dative bonds, the strength of the bond is highly dependent on the energy match between the highest occupied molecular orbital (HOMO) of the NHC ligand and that of the metal ion. Because of their versatility, the coordination chemistry of NHC ligands with was explored with almost all transition metal ions. Other than the transition metals, NHCs are also capable of establishing a chemical bond with the main group elements. The advances in the catalytic applications of the NHC ligands linked with a second tether are discussed. For clarity, more frequently targeted catalytic reactions are considered first. Carbon-carbon coupling reactions, transfer hydrogenation of alkenes and carbonyl compounds, ketone hydrosilylation, and chiral catalysis are among highly popular reactions. Areas where the efficacy of the NHC based catalytic systems were explored to a lesser extent include CO2 reduction, C-H borylation, alkyl amination, and hydroamination reactions. Furthermore, the synthesis and applications of transition metal complexes are covered.
Keywords: N-heterocyclic carbenes; catalysis; chiral; coupling reactions; polymerization; transfer hydrogenation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

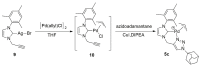



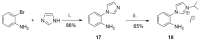


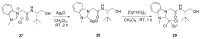





















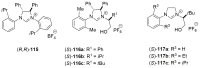

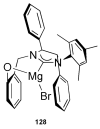






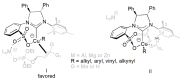
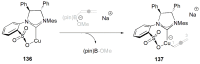







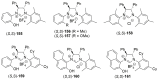
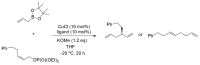
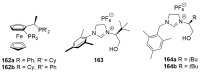
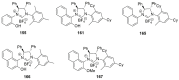






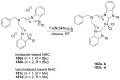




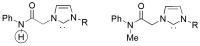



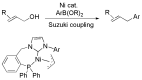


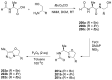



























References
-
- Arduengo A.J., Harlow R.L., Kline M. A stable crystalline carbene. J. Am. Chem. Soc. 1991;113:361–363. doi: 10.1021/ja00001a054. - DOI
-
- Herrmann W.A., Köcher C., Gooßen L.J., Artus G.R.J. Heterocyclic Carbenes: A High-Yielding Synthesis of Novel, Functionalized N-Heterocyclic Carbenes in Liquid Ammonia. Chem. Eur. J. 1996;2:1627–1636. doi: 10.1002/chem.19960021222. - DOI
-
- Edworthy I.S., Rodden M., Mungur S.A., Davis K.M., Blake A.J., Wilson C., Schröder M., Arnold P.L. Silver alkoxide and amino N-heterocyclic carbenes; syntheses and crystal structures. J. Organomet. Chem. 2005;690:5710–5719. doi: 10.1016/j.jorganchem.2005.07.063. - DOI
-
- Becker E., Stingl V., Dazinger G., Mereiter K., Kirchner K. Migratory Insertion of Acetylene in N-Heterocyclic Carbene Complexes of Ruthenium: Formation of (Ruthenocenylmethyl)imidazolium Salts. Organometallics. 2007;26:1531–1535. doi: 10.1021/om060990i. - DOI
Publication types
LinkOut - more resources
Full Text Sources

