Synthesis, Pharmacological Evaluation, and Computational Studies of Cyclic Opioid Peptidomimetics Containing β3-Lysine
- PMID: 35011383
- PMCID: PMC8747000
- DOI: 10.3390/molecules27010151
Synthesis, Pharmacological Evaluation, and Computational Studies of Cyclic Opioid Peptidomimetics Containing β3-Lysine
Abstract
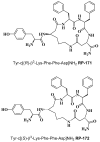
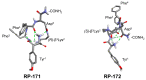
Our formerly described pentapeptide opioid analog Tyr-c[D-Lys-Phe-Phe-Asp]NH2 (designated RP-170), showing high affinity for the mu (MOR) and kappa (KOR) opioid receptors, was much more stable than endomorphine-2 (EM-2) in the rat brain homogenate and displayed remarkable antinociceptive activity after central (intracerebroventricular) and peripheral (intravenous ) administration. In this report, we describe the further modification of this analog, which includes the incorporation of a β3-amino acid, (R)- and (S)-β3-Lys, instead of D-Lys in position 2. The influence of such replacement on the biological properties of the obtained analogs, Tyr-c[(R)-β3-Lys-Phe-Phe-Asp]NH2 (RP-171) and Tyr-c[(S)-β3-Lys-Phe-Phe-Asp]NH2, (RP-172), was investigated in vitro. Receptor radiolabeled displacement and functional calcium mobilization assays were performed to measure binding affinity and receptor activation of the new analogs. The obtained data revealed that only one of the diastereoisomeric peptides, RP-171, was able to selectively bind and activate MOR. Molecular modeling (docking and molecular dynamics (MD) simulations) suggests that both compounds should be accommodated in the MOR binding site. However, in the case of the inactive isomer RP-172, fewer hydrogen bonds, as well as instability of the canonical ionic interaction to Asp147, could explain its very low MOR affinity.
Keywords: functional assay; opioid receptors; peptide synthesis; receptor binding studies; β-amino acids.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

