Thallium(I) Tropolonates: Synthesis, Structure, Spectral Characteristics, and Antimicrobial Activity Compared to Lead(II) and Bismuth(III) Analogues
- PMID: 35011415
- PMCID: PMC8746424
- DOI: 10.3390/molecules27010183
Thallium(I) Tropolonates: Synthesis, Structure, Spectral Characteristics, and Antimicrobial Activity Compared to Lead(II) and Bismuth(III) Analogues
Abstract
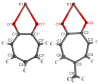
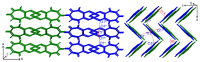
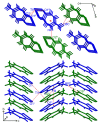
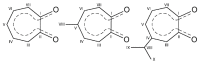
Synthesis, single-crystal X-ray determination diffraction and FT-IR, NMR (1H, 13C, 19F and 205Tl), UV-vis, and luminescence spectra characteristics were described for series of thallium(I) compounds: thallium(I) triflate (Tl(OTf)), 1:1 co-crystals of thallium(I) triflate and tropolone (Htrop), Tl(OTf)·Htrop, as well as simple thallium(I) chelates: Tl(trop) (1), Tl(5-metrop) (2), Tl(hino) (3), with Htrop, 5-methyltropolone (5-meHtrop), 4-isopropyltropolone (hinokitiol, Hhino), respectively, and additionally more complex {Tl@[Tl(hino)]6}(OTf) (4) compound. Comparison of their antimicrobial activity with selected lead(II) and bismuth(III) analogs and free ligands showed that only bismuth(III) complexes demonstrated significant antimicrobial activity, from two- to fivefold larger than the free ligands.
Keywords: 5-methyltropolone; antimicrobial activity; crystal structure; hinokitiol; thallium(I) complexes; thallium(I) triflate; tropolone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Saniewski M., Horbowicz M., Kanlayanarat S. The biological activities of troponoids and their use in agriculture. J. Hort. Res. 2014;22:5–19. doi: 10.2478/johr-2014-0001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

