Investigation of Metastable Low Dimensional Halometallates
- PMID: 35011512
- PMCID: PMC8746344
- DOI: 10.3390/molecules27010280
Investigation of Metastable Low Dimensional Halometallates
Abstract
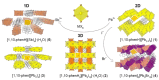
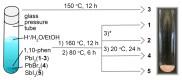
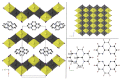
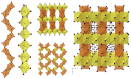
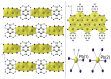
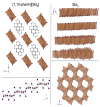
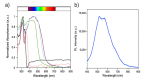
The solvothermal synthesis, structure determination and optical characterization of five new metastable halometallate compounds, [1,10-phenH][Pb3.5I8] (1), [1,10-phenH2][Pb5I12]·(H2O) (2), [1,10-phen][Pb2I4] (3), [1,10-phen]2[Pb5Br10] (4) and [1,10-phenH][SbI4]·(H2O) (5), are reported. The materials exhibit rich structural diversity and exhibit structural dimensionalities that include 1D chains, 2D sheets and 3D frameworks. The optical spectra of these materials are consistent with bandgaps ranging from 2.70 to 3.44 eV. We show that the optical behavior depends on the structural dimensionality of the reported materials, which are potential candidates for semiconductor applications.
Keywords: dimensional reduction; halometallate; hydrothermal synthesis.
Conflict of interest statement
The authors declare no known competing financial interest or conflict of interest.
Figures



References
-
- Jonderian A., Ting M., McCalla E. Metastability in Li–La–Ti–O Perovskite Materials and Its Impact on Ionic Conductivity. Chem. Mater. 2021;33:4792–4804. doi: 10.1021/acs.chemmater.1c01490. - DOI
-
- Li W., Wang Z., Deschler F., Gao S., Friend R.H., Cheetham A.K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017;2:16099. doi: 10.1038/natrevmats.2016.99. - DOI
-
- Lin H., Zhou C., Tian Y., Siegrist T., Ma B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018;3:54–62. doi: 10.1021/acsenergylett.7b00926. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

