Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3- d]pyrimidines as Microtubule Targeting Agents
- PMID: 35011550
- PMCID: PMC8747035
- DOI: 10.3390/molecules27010321
Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3- d]pyrimidines as Microtubule Targeting Agents
Abstract
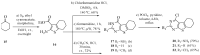
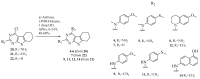
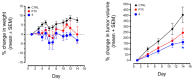
A series of eleven 4-substituted 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines were designed and synthesized and their biological activities were evaluated. Synthesis involved the Gewald reaction to synthesize ethyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate ring, and SNAr reactions. Compound 4 was 1.6- and ~7-fold more potent than the lead compound 1 in cell proliferation and microtubule depolymerization assays, respectively. Compounds 4, 5 and 7 showed the most potent antiproliferative effects (IC50 values < 40 nM), while compounds 6, 8, 10, 12 and 13 had lower antiproliferative potencies (IC50 values of 53-125 nM). Additionally, compounds 4-8, 10 and 12-13 circumvented Pgp and βIII-tubulin mediated drug resistance, mechanisms that diminish the clinical efficacy of paclitaxel (PTX). In the NCI-60 cell line panel, compound 4 exhibited an average GI50 of ~10 nM in the 40 most sensitive cell lines. Compound 4 demonstrated statistically significant antitumor effects in a murine MDA-MB-435 xenograft model.
Keywords: Gewald reaction; colchicine site; microtubule targeting agents; microtubules.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Figures

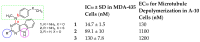


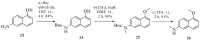
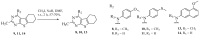
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

