Crimean-Congo Hemorrhagic Fever Virus Past Infections Are Associated with Two Innate Immune Response Candidate Genes in Dromedaries
- PMID: 35011568
- PMCID: PMC8750074
- DOI: 10.3390/cells11010008
Crimean-Congo Hemorrhagic Fever Virus Past Infections Are Associated with Two Innate Immune Response Candidate Genes in Dromedaries
Abstract
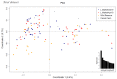
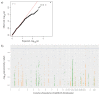
Dromedaries are an important livestock, used as beasts of burden and for meat and milk production. However, they can act as an intermediate source or vector for transmitting zoonotic viruses to humans, such as the Middle East respiratory syndrome coronavirus (MERS-CoV) or Crimean-Congo hemorrhagic fever virus (CCHFV). After several outbreaks of CCHFV in the Arabian Peninsula, recent studies have demonstrated that CCHFV is endemic in dromedaries and camel ticks in the United Arab Emirates (UAE). There is no apparent disease in dromedaries after the bite of infected ticks; in contrast, fever, myalgia, lymphadenopathy, and petechial hemorrhaging are common symptoms in humans, with a case fatality ratio of up to 40%. We used the in-solution hybridization capture of 100 annotated immune genes to genotype 121 dromedaries from the UAE tested for seropositivity to CCHFV. Through univariate linear regression analysis, we identified two candidate genes belonging to the innate immune system: FCAR and CLEC2B. These genes have important functions in the host defense against viral infections and in stimulating natural killer cells, respectively. This study opens doors for future research into immune defense mechanisms in an enzootic host against an important zoonotic disease.
Keywords: Camelus dromedarius; Old World camel; in-solution hybridization capture; tick; vector-borne infection; zoonosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L., Hoffmann M., Pöhlmann S., et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. 2020;181:1004–1015.e15. doi: 10.1016/j.cell.2020.04.031. - DOI - PMC - PubMed
-
- Koenig P.-A., Das H., Liu H., Kümmerer B.M., Gohr F.N., Jenster L.-M., Schiffelers L.D.J., Tesfamariam Y.M., Uchima M., Wuerth J.D., et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021;371:eabe6230. doi: 10.1126/science.abe6230. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

