Impact of STING Inflammatory Signaling during Intracellular Bacterial Infections
- PMID: 35011636
- PMCID: PMC8750390
- DOI: 10.3390/cells11010074
Impact of STING Inflammatory Signaling during Intracellular Bacterial Infections
Abstract
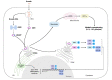
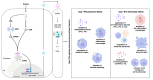
The early detection of bacterial pathogens through immune sensors is an essential step in innate immunity. STING (Stimulator of Interferon Genes) has emerged as a key mediator of inflammation in the setting of infection by connecting pathogen cytosolic recognition with immune responses. STING detects bacteria by directly recognizing cyclic dinucleotides or indirectly by bacterial genomic DNA sensing through the cyclic GMP-AMP synthase (cGAS). Upon activation, STING triggers a plethora of powerful signaling pathways, including the production of type I interferons and proinflammatory cytokines. STING activation has also been associated with the induction of endoplasmic reticulum (ER) stress and the associated inflammatory responses. Recent reports indicate that STING-dependent pathways participate in the metabolic reprogramming of macrophages and contribute to the establishment and maintenance of a robust inflammatory profile. The induction of this inflammatory state is typically antimicrobial and related to pathogen clearance. However, depending on the infection, STING-mediated immune responses can be detrimental to the host, facilitating bacterial survival, indicating an intricate balance between immune signaling and inflammation during bacterial infections. In this paper, we review recent insights regarding the role of STING in inducing an inflammatory profile upon intracellular bacterial entry in host cells and discuss the impact of STING signaling on the outcome of infection. Unraveling the STING-mediated inflammatory responses can enable a better understanding of the pathogenesis of certain bacterial diseases and reveal the potential of new antimicrobial therapy.
Keywords: STING; bacteria; cyclic dinucleotides; infection; inflammation; type I interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- 406883/2018-1/National Council for Scientific and Technological Development
- 00140-16/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- APQ 01945/17/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 465229/2014-0/National Council for Scientific and Technological Development
- R01 AI116453/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

