Interleukin-8 in Melanoma Pathogenesis, Prognosis and Therapy-An Integrated View into Other Neoplasms and Chemokine Networks
- PMID: 35011682
- PMCID: PMC8750532
- DOI: 10.3390/cells11010120
Interleukin-8 in Melanoma Pathogenesis, Prognosis and Therapy-An Integrated View into Other Neoplasms and Chemokine Networks
Abstract
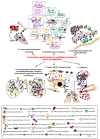
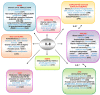
Cutaneous melanoma accounts for only about 7% of skin cancers but is causing almost 90% of deaths. Melanoma cells have a distinct repertoire of mutations from other cancers, a high plasticity and degree of mimicry toward vascular phenotype, stemness markers, versatility in evading and suppress host immune control. They exert a significant influence on immune, endothelial and various stromal cells which form tumor microenvironment. The metastatic stage, the leading cause of mortality in this neoplasm, is the outcome of a complex, still poorly understood, cross-talk between tumor and other cell phenotypes. There is accumulating evidence that Interleukin-8 (IL-8) is emblematic for advanced melanomas. This work aimed to present an updated status of IL-8 in melanoma tumor cellular complexity, through a comprehensive analysis including data from other chemokines and neoplasms. The multiple processes and mechanisms surveyed here demonstrate that IL-8 operates following orchestrated programs within signaling webs in melanoma, stromal and vascular cells. Importantly, the yet unknown molecularity regulating IL-8 impact on cells of the immune system could be exploited to overturn tumor fate. The molecular and cellular targets of IL-8 should be brought into the attention of even more intense scientific exploration and valorization in the therapeutical management of melanoma.
Keywords: CXCL8; CXCR1; CXCR2; chemokines; chemokines in tumor progression; interleukin-8.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

