Pathologic Proteolytic Processing of N-Cadherin as a Marker of Human Fibrotic Disease
- PMID: 35011717
- PMCID: PMC8750447
- DOI: 10.3390/cells11010156
Pathologic Proteolytic Processing of N-Cadherin as a Marker of Human Fibrotic Disease
Abstract
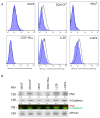
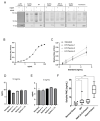
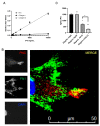
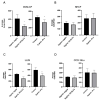
Prior research has implicated the involvement of cell adhesion molecule N-cadherin in tissue fibrosis and remodeling. We hypothesize that anomalies in N-cadherin protein processing are involved in pathological fibrosis. Diseased tissues associated with fibrosis of the heart, lung, and liver were probed for the precursor form of N-cadherin, pro-N-cadherin (PNC), by immunohistochemistry and compared to healthy tissues. Myofibroblast cell lines were analyzed for cell surface pro-N-cadherin by flow cytometry and immunofluorescent microscopy. Soluble PNC products were immunoprecipitated from patient plasmas and an enzyme-linked immunoassay was developed for quantification. All fibrotic tissues examined show aberrant PNC localization. Cell surface PNC is expressed in myofibroblast cell lines isolated from cardiomyopathy and idiopathic pulmonary fibrosis but not on myofibroblasts isolated from healthy tissues. PNC is elevated in the plasma of patients with cardiomyopathy (p ≤ 0.0001), idiopathic pulmonary fibrosis (p ≤ 0.05), and nonalcoholic fatty liver disease with cirrhosis (p ≤ 0.05). Finally, we have humanized a murine antibody and demonstrate that it significantly inhibits migration of PNC expressing myofibroblasts. Collectively, the aberrant localization of PNC is observed in all fibrotic tissues examined in our study and our data suggest a role for cell surface PNC in the pathogenesis of fibrosis.
Keywords: N-cadherin; cirrhosis; fibronectin; fibrosis; heart failure; myofibroblasts; pro-N-cadherin; pulmonary fibrosis.
Conflict of interest statement
See patent disclosure. The authors declare no further conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

