Health-Related Quality of Life Outcomes in Patients with Myelodysplastic Syndromes with Ring Sideroblasts Treated with Luspatercept in the MEDALIST Phase 3 Trial
- PMID: 35011768
- PMCID: PMC8745777
- DOI: 10.3390/jcm11010027
Health-Related Quality of Life Outcomes in Patients with Myelodysplastic Syndromes with Ring Sideroblasts Treated with Luspatercept in the MEDALIST Phase 3 Trial
Abstract
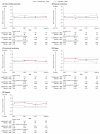
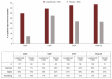
Patients with myelodysplastic syndromes (MDS) often experience chronic anemia and long-term red blood cell transfusion dependence associated with significant burden on clinical and health-related quality of life (HRQoL) outcomes. In the MEDALIST trial (NCT02631070), luspatercept significantly reduced transfusion burden in patients with lower-risk MDS who had ring sideroblasts and were refractory to, intolerant to, or ineligible for prior treatment with erythropoiesis-stimulating agents. We evaluated the effect of luspatercept on HRQoL in patients enrolled in MEDALIST using the EORTC QLQ-C30 and the QOL-E questionnaire. Change in HRQoL was assessed every 6 weeks in patients receiving luspatercept with best supportive care (+ BSC) and placebo + BSC from baseline through week 25. No clinically meaningful within-group changes and between-group differences across all domains of the EORTC QLQ-C30 and QOL-E were observed. On one item of the QOL-E MDS-specific disturbances domain, patients treated with luspatercept reported marked improvements in their daily life owing to the reduced transfusion burden, relative to placebo. Taken together with previous reports of luspatercept + BSC reducing transfusion burden in patients from baseline through week 25 in MEDALIST, these results suggest luspatercept may offer a treatment option for patients that reduces transfusion burden while providing stability in HRQoL.
Keywords: luspatercept; myelodysplastic syndromes; quality of life; transfusion dependence.
Conflict of interest statement
Ester N. Oliva: consultancy, honoraria for/from Amgen, BMS, and Novartis; honoraria, patents, royalties, speakers’ bureau from/for BMS. Uwe Platzbecker: consultancy, honoraria for/from BMS, Janssen, and Novartis; research funding from Amgen, Janssen, Merck, and Novartis. Guillermo Garcia-Manero: consultancy for Astex Pharmaceuticals, BMS, Genentech, Helsinn Therapeutics; research funding from AbbVie, Amphivena Therapeutics, Aprea, Astex Pharmaceuticals, BMS, Curis, Forty Seven, Genentech, Helsinn Therapeutics, H3 Biomedicine, Janssen, Merck, Novartis, Onconova. Ghulam J. Mufti: research funding from Aplastic Anaemia Trust, Bloodwise UK/Leukaemia and Lymphoma Research Programme, BMS, Cancer Research UK, Life Arc, and Novartis. Valeria Santini: research funding from Janssen; honoraria from BMS, Johnson & Johnson, and Novartis; consultancy for Acceleron Pharma, BMS, Menarini, and Novartis; membership on an entity’s board of directors or advisory committees for Pfizer and Takeda. Mikkael A. Sekeres: membership on an entity’s board of directors or advisory committees for BMS, Millennium/Takeda, and Novartis. Rami S. Komrokji: honoraria from AbbVie, Acceleron Pharma, Agios, BMS, Geron, Jazz Pharmaceuticals, and Novartis; speakers’ bureau for BMS and Jazz Pharmaceuticals. Jeevan K. Shetty, George Zhang, and Xianwei Ha: employment at BMS. Derek Tang and Jennifer Lord-Bessen: employment, equity ownership at BMS. Rodrigo Ito: former employment at BMS during time of study; current employment at Eli Lilly and Company; equity ownership at BMS, Eli Lilly and Company. Shien Guo: consultancy for BMS, Gilead, Janssen. Weigin Liao: consultancy for BMS. Jay T. Backstrom: employment, equity ownership at Acceleron Pharma; equity ownership at BMS. Pierre Fenaux: honoraria from BMS.
Figures


References
-
- Steensma D.P., Heptinstall K.V., Johnson V.M., Novotny P.J., Sloan J.A., Camoriano J.K., Niblack J., Bennett J.M., Mesa R.A. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): Results of a large internet-based survey. Leuk. Res. 2008;32:691–698. doi: 10.1016/j.leukres.2007.10.015. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

