Waning Humoral Response 3 to 6 Months after Vaccination with the SARS-COV-2 BNT162b2 mRNA Vaccine in Dialysis Patients
- PMID: 35011801
- PMCID: PMC8745040
- DOI: 10.3390/jcm11010064
Waning Humoral Response 3 to 6 Months after Vaccination with the SARS-COV-2 BNT162b2 mRNA Vaccine in Dialysis Patients
Abstract
Background and objectives: The short-term reported antibody response to SARS-COV-2 vaccination in dialysis patients is high, with a seroconversion response rate up to 97%. Data on the long-term durability of this response are scarce. Our objective was to characterize the long-term anti-spike antibody level in dialysis patients.
Design, setting, participants, and measurements: In an observational study, we measured SARS-COV-2 anti-spike antibody levels in dialysis patients who completed 2 doses of the BNT162b2 mRNA SAR S-COV-2 vaccine at 1, 3 and 6 months after the second vaccine dose. We compared the response to dialysis patients who were infected with COVD-19 and to a control group of healthcare-employees.
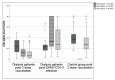
Results: One hundred and forty-two dialysis patients who had been vaccinated (ages 64 ± 11.9 years, 61% male), 33 dialysis patients who had COVID-19 infection (ages 54 ± 14.3 years, 55% male) and 104 individuals in the control group (ages 50 ± 12.2 years, 44% male) were included. The response rate in the vaccinated dialysis patients was 94%, 78% and 73% at 1, 3 and 6 months after the second vaccine dose. In the COVID-19 infected dialysis group and in the control group, the response rate remained at 100% over 6 months. The percentage of change in antibody levels between one and 6 months was -66% in the vaccinated dialysis group, -28% in the control group (p < 0.001) and +48% in dialysis patients who had been infected with COVID-19 (p < 0.001). A non-responder status at 6 months was associated with a lower albumin level. No serious adverse events following vaccination were reported. In conclusion: the initially high response rate to the BNT162b2 vaccine in dialysis patients decreases rapidly. Our results indicate that an early booster (3rd) dose, at three months after the second dose, may be advised for this population to preserve the humoral immunity.
Keywords: BNT162b2 mRNA vaccine; SAR S-COV-2; dialysis; end stage renal disease; waning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hilbrands L.B., Duivenvoorden R., Vart P., Franssen C.F.M., Hemmelder M.H., Jager K.J., Kieneker L.M., Noordzij M., Pena M.J., de Vries H., et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. - DOI - PMC - PubMed
-
- Hsu C.M., Weiner D.E., Aweh G., Miskulin D.C., Manley H.J., Stewart C., Ladik V., Hosford J., Lacson E.C., Johnson D.S., et al. COVID-19 among US dialysis patients: Risk factors and outcomes from national dialysis provider. Am. J. Kidney Dis. 2021;77:748–756. doi: 10.1053/j.ajkd.2021.01.003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

