Detection of SARS-CoV-2 RNA in Urine by RT-LAMP: A Very Rare Finding
- PMID: 35011899
- PMCID: PMC8745354
- DOI: 10.3390/jcm11010158
Detection of SARS-CoV-2 RNA in Urine by RT-LAMP: A Very Rare Finding
Abstract
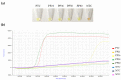
Detection of SARS-CoV-2 is routinely performed in naso/oropharyngeal swabs samples from patients via RT-qPCR. The RT-LAMP technology has also been used for viral RNA detection in respiratory specimens with both high sensitivity and specificity. Recently, we developed a novel RT-LAMP test for SARS-CoV-2 RNA detection in nasopharyngeal swab specimens (named, N15-RT-LAMP) that can be performed as a single-tube colorimetric method, in a real-time platform, and as dry-LAMP. To date, there has been very little success in detecting SARS-CoV-2 RNA in urine by RT-qPCR, and the information regarding urine viral excretion is still scarce and not comprehensive. Here, we tested our N15-RT-LAMP on the urine of 300 patients admitted to the Hospital of Salamanca, Spain with clinical suspicion of COVID-19, who had a nasopharyngeal swab RT-qPCR-positive (n = 100), negative (n = 100), and positive with disease recovery (n = 100) result. The positive group was also tested by RT-qPCR for comparison to N15-RT-LAMP. Only a 4% positivity rate was found in the positive group via colorimetric N15-RT-LAMP and 2% via RT-qPCR. Our results are consistent with those obtained in other studies that the presence of SARS-CoV-2 RNA in urine is a very rare finding. The absence of SARS-CoV-2 RNA in urine in the recovered patients might suggest that the urinary route is very rarely used for viral particle clearance.
Keywords: COVID-19; RT-LAMP; RT-qPCR; SARS-CoV-2; molecular diagnostics; urine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
RT-LAMP Multicenter Study for SARS-CoV-2 Genome Molecular Detection in Brazilian Swab and Saliva Samples.Diagnostics (Basel). 2023 Jan 6;13(2):210. doi: 10.3390/diagnostics13020210. Diagnostics (Basel). 2023. PMID: 36673025 Free PMC article.
-
Rapid, sensitive, and specific detection of SARS-CoV-2 in nasopharyngeal swab samples of suspected patients using a novel one-step loop-mediated isothermal amplification (one-step LAMP) technique.BMC Microbiol. 2023 Mar 7;23(1):63. doi: 10.1186/s12866-023-02806-z. BMC Microbiol. 2023. PMID: 36882699 Free PMC article.
-
Isothermal amplification and fluorescent detection of SARS-CoV-2 and SARS-CoV-2 variant virus in nasopharyngeal swabs.PLoS One. 2021 Sep 17;16(9):e0257563. doi: 10.1371/journal.pone.0257563. eCollection 2021. PLoS One. 2021. PMID: 34534259 Free PMC article.
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
Automated sample-to-answer centrifugal microfluidic system for rapid molecular diagnostics of SARS-CoV-2.Lab Chip. 2022 Aug 23;22(17):3157-3171. doi: 10.1039/d2lc00242f. Lab Chip. 2022. PMID: 35670202
Cited by
-
First field and laboratory evaluation of LAMP assay for malaria diagnosis in Cubal, Angola.Parasit Vectors. 2023 Oct 3;16(1):343. doi: 10.1186/s13071-023-05942-7. Parasit Vectors. 2023. PMID: 37789462 Free PMC article.
-
Molecular Diagnosis of COVID-19; Biosafety and Pre-analytical Recommendations.Iran J Pathol. 2023 Summer;18(3):244-256. doi: 10.30699/IJP.2023.1988405.3061. Epub 2023 Jul 16. Iran J Pathol. 2023. PMID: 37942195 Free PMC article. Review.
-
SARS-CoV-2 in semen: a multicenter prospective study and literature review.Basic Clin Androl. 2024 Dec 2;34(1):24. doi: 10.1186/s12610-024-00236-z. Basic Clin Androl. 2024. PMID: 39617887 Free PMC article.
-
The Future of Point-of-Care Nucleic Acid Amplification Diagnostics after COVID-19: Time to Walk the Walk.Int J Mol Sci. 2022 Nov 15;23(22):14110. doi: 10.3390/ijms232214110. Int J Mol Sci. 2022. PMID: 36430586 Free PMC article. Review.
-
A rapid, specific, extraction-less, and cost-effective RT-LAMP test for the detection of SARS-CoV-2 in clinical specimens.PLoS One. 2022 Apr 11;17(4):e0266703. doi: 10.1371/journal.pone.0266703. eCollection 2022. PLoS One. 2022. PMID: 35404944 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

