The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa
- PMID: 35011927
- PMCID: PMC8745326
- DOI: 10.3390/jcm11010186
The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa
Abstract
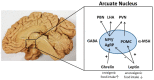
Eating behavior is controlled by hypothalamic circuits in which agouti-related peptide-expressing neurons when activated in the arcuate nucleus, promote food intake while pro-opiomelanocortin-producing neurons promote satiety. The respective neurotransmitters signal to other parts of the hypothalamus such as the paraventricular nucleus as well as several extra-hypothalamic brain regions to orchestrate eating behavior. This complex process of food intake may be influenced by glia cells, in particular astrocytes and microglia. Recent studies showed that GFAP+ astrocyte cell density is reduced in the central nervous system of an experimental anorexia nervosa model. Anorexia nervosa is an eating disorder that causes, among the well-known somatic symptoms, brain volume loss which was associated with neuropsychological deficits while the underlying pathophysiology is unknown. In this review article, we summarize the findings of glia cells in anorexia nervosa animal models and try to deduce which role glia cells might play in the pathophysiology of eating disorders, including anorexia nervosa. A better understanding of glia cell function in the regulation of food intake and eating behavior might lead to the identification of new drug targets.
Keywords: anorexia nervosa; astrocyte; glia cells; hypothalamus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhang Y., Chua S., Jr. Leptin function and regulation. Compr. Physiol. 2011;8:351–369. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

