Computational Study of Catalytic Urethane Formation
- PMID: 35012031
- PMCID: PMC8747140
- DOI: 10.3390/polym14010008
Computational Study of Catalytic Urethane Formation
Abstract
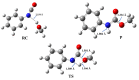
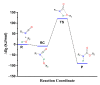
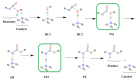
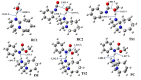
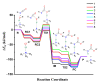
Polyurethanes (PUs) are widely used in different applications, and thus various synthetic procedures including one or more catalysts are applied to prepare them. For PU foams, the most important catalysts are nitrogen-containing compounds. Therefore, in this work, the catalytic effect of eight different nitrogen-containing catalysts on urethane formation will be examined. The reactions of phenyl isocyanate (PhNCO) and methanol without and in the presence of catalysts have been studied and discussed using the G3MP2BHandHLYP composite method. The solvent effects have also been considered by applying the SMD implicit solvent model. A general urethane formation mechanism has been proposed without and in the presence of the studied catalysts. The proton affinities (PA) were also examined. The barrier height of the reaction significantly decreased (∆E0 > 100 kJ/mol) in the presence of the studied catalysts, which proves the important effect they have on urethane formation. The achieved results can be applied in catalyst design and development in the near future.
Keywords: DFT; catalyst-free; catalysts; composite method; proton affinities; urethane formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Szycher M. Szycher’s Handbook of Polyurethanes. CRC Press; Boca Raton, FL, USA: 2012. Structure–Property Relations in Polyurethanes.
-
- Szycher M. Handbook of Polyurethanes. CRC Press; Boca Raton, FL, USA: 1938. p. 142.
-
- Cavaco L.I. In: Polyurethanes Properies, Structure and Applicatiovs. Melo J.A., editor. Nova Science Publishers, Inc.; New York, NY, USA: 2012.
-
- Sabrina S.S.A., Denilson A.S., Danielle M.A. Physico-Chemical Analysis of Flexible Polyurethane Foams Containing Commercial Calcium Carbonate. Mater. Res. 2008;11:433–438. doi: 10.1590/s1516-14392008000400009. - DOI
-
- Akindoyo J.O., Beg M.D.H., Ghazali S., Islam M.R., Jeyaratnam N., Yuvaraj A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016;6:114453–114482. doi: 10.1039/C6RA14525F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

