Immunoprotection against Cryptococcosis Offered by Znf2 Depends on Capsule and the Hyphal Morphology
- PMID: 35012334
- PMCID: PMC8749420
- DOI: 10.1128/mbio.02785-21
Immunoprotection against Cryptococcosis Offered by Znf2 Depends on Capsule and the Hyphal Morphology
Abstract
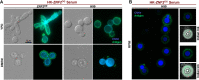
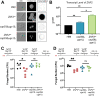
Systemic cryptococcosis is fatal without treatment. Globally, this disease kills 180,000 of the 225,000 infected people each year, even with the use of antifungal therapies. Currently, there is no vaccine to prevent cryptococcosis. Previously, we discovered that Znf2, a morphogenesis regulator that directs Cryptococcus yeast-to-hyphal transition, profoundly affects cryptococcal interaction with the host-overexpression of ZNF2 drives filamentous growth, attenuates cryptococcal virulence, and elicits protective host immune responses. Importantly, immunization with cryptococcal cells overexpressing ZNF2, either in live or heat-inactivated form, offers significant protection to the host from a subsequent challenge by the otherwise lethal wild-type H99 strain. We hypothesize that cellular components enriched in ZNF2oe cells are immunoprotective. Here, we discovered that serum from protected animals vaccinated with inactivated ZNF2oe cells recognizes cryptococcal antigens that reside within the capsule. Consistently, capsule is required for immunoprotection offered by ZNF2oe cells. Interestingly, the serum from protective animals recognizes antigens in both wild-type yeast cells and ZNF2oe cells, with higher abundance in the latter. Consequently, even the heat-inactivated wild-type cells become immunoprotective with an increased vaccination dose. We also found that disruption of a chromatin remodeling factor Brf1, which is important for initiation of filamentation by Znf2, reduces the antigen level in ZNF2oe cells. Deletion of BRF1 drastically reduces the protective effect of ZNF2oe cells in both live and heat-killed forms even though the ZNF2oebrf1Δ strain itself is avirulent. Collectively, our findings underscore the importance of identifying the subset of cryptococcal surface factors that are beneficial in host protection. IMPORTANCE Cryptococcosis claims close to 200,000 lives annually. There is no vaccine clinically available for this fungal disease. Many avirulent mutant strains do not provide protection against cryptococcosis. We previously discovered that hyphal ZNF2oe strains elicit protective host immune responses both in the live and heat-inactivated forms. Here we seek to understand the mechanism underlying the host protection provided by ZNF2oe cells. We discovered increased accumulation of antigens located within the caspusle of ZNF2oe cells and consequently the requirement of the capsule for ZNF2oe strain-elicited host protection. Furthermore, genetically blocking the ability of ZNF2oe cells to grow in the hyphal form significantly reduces antigen accumulation and impairs the ability of ZNF2oe strain to provide host protection. Our findings highlight the importance of identifying the Znf2-regulated capsular surface factors that are fundamental in host protection.
Keywords: Cryptococcus neoformans; antigens; capsule; immunofluorescence; morphogenesis; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. - DOI - PMC - PubMed
-
- Chaiwarith R, Vongsanim S, Supparatpinyo K. 2014. Cryptococcal meningitis in HIV-infected patients at Chiang Mai University Hospital: a retrospective study. Southeast Asian J Trop Med Public Health 45:636–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
