Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology
- PMID: 35012610
- PMCID: PMC8744064
- DOI: 10.1186/s13045-021-01222-y
Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology
Abstract
Background: Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) through direct lysis of infected lung epithelial cells, which releases damage-associated molecular patterns and induces a pro-inflammatory cytokine milieu causing systemic inflammation. Anti-viral and anti-inflammatory agents have shown limited therapeutic efficacy. Soluble CD24 (CD24Fc) blunts the broad inflammatory response induced by damage-associated molecular patterns via binding to extracellular high mobility group box 1 and heat shock proteins, as well as regulating the downstream Siglec10-Src homology 2 domain-containing phosphatase 1 pathway. A recent randomized phase III trial evaluating CD24Fc for patients with severe COVID-19 (SAC-COVID; NCT04317040) demonstrated encouraging clinical efficacy.
Methods: Using a systems analytical approach, we studied peripheral blood samples obtained from patients enrolled at a single institution in the SAC-COVID trial to discern the impact of CD24Fc treatment on immune homeostasis. We performed high dimensional spectral flow cytometry and measured the levels of a broad array of cytokines and chemokines to discern the impact of CD24Fc treatment on immune homeostasis in patients with COVID-19.
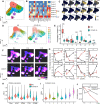
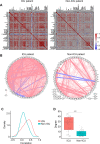
Results: Twenty-two patients were enrolled, and the clinical characteristics from the CD24Fc vs. placebo groups were matched. Using high-content spectral flow cytometry and network-level analysis, we found that patients with severe COVID-19 had systemic hyper-activation of multiple cellular compartments, including CD8+ T cells, CD4+ T cells, and CD56+ natural killer cells. Treatment with CD24Fc blunted this systemic inflammation, inducing a return to homeostasis in NK and T cells without compromising the anti-Spike protein antibody response. CD24Fc significantly attenuated the systemic cytokine response and diminished the cytokine coexpression and network connectivity linked with COVID-19 severity and pathogenesis.
Conclusions: Our data demonstrate that CD24Fc rapidly down-modulates systemic inflammation and restores immune homeostasis in SARS-CoV-2-infected individuals, supporting further development of CD24Fc as a novel therapeutic against severe COVID-19.
Keywords: CD24Fc; COVID-19; Cytokine score; Immunophenotyping; Soluble CD24.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Update of
-
TREATMENT WITH SOLUBLE CD24 ATTENUATES COVID-19-ASSOCIATED SYSTEMIC IMMUNOPATHOLOGY.medRxiv [Preprint]. 2021 Sep 16:2021.08.18.21262258. doi: 10.1101/2021.08.18.21262258. medRxiv. 2021. Update in: J Hematol Oncol. 2022 Jan 10;15(1):5. doi: 10.1186/s13045-021-01222-y. PMID: 34462760 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

