Functional impact and targetability of PI3KCA, GNAS, and PTEN mutations in a spindle cell rhabdomyosarcoma with MYOD1 L122R mutation
- PMID: 35012940
- PMCID: PMC8744497
- DOI: 10.1101/mcs.a006140
Functional impact and targetability of PI3KCA, GNAS, and PTEN mutations in a spindle cell rhabdomyosarcoma with MYOD1 L122R mutation
Erratum in
-
Corrigendum: Functional impact and targetability of PI3KCA, GNAS, and PTEN mutations in a spindle cell rhabdomyosarcoma with MYOD1 L122R mutation.Cold Spring Harb Mol Case Stud. 2022 Apr 28;8(3):a006216. doi: 10.1101/mcs.a006216. Print 2022 Apr. Cold Spring Harb Mol Case Stud. 2022. PMID: 35483883 Free PMC article. No abstract available.
Abstract
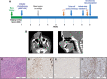
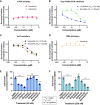
Spindle cell/sclerosing rhabdomyosarcoma (ssRMS) is a rare subtype of rhabdomyosarcoma, commonly harboring a gain-of-function L122R mutation in the muscle-specific master transcription factor MYOD1. MYOD1-mutated ssRMS is almost invariably fatal, and development of novel therapeutic approaches based on the biology of the disease is urgently needed. MYOD1 L122R affects the DNA-binding domain and is believed to confer MYC-like properties to MYOD1, driving oncogenesis. Moreover, the majority of the MYOD1-mutated ssRMS harbor additional alterations activating the PI3K/AKT pathway. It is postulated that the PI3K/AKT pathway cooperates with MYOD1 L122R. To address this biological entity, we established and characterized a new patient-derived ssRMS cell line OHSU-SARC001, harboring MYOD1 L122R as well as alterations in PTEN, PIK3CA, and GNAS We explored the functional impact of these aberrations on oncogenic signaling with gain-of-function experiments in C2C12 murine muscle lineage cells. These data reveal that PIK3CAI459_T462del, the novel PIK3CA variant discovered in this patient specimen, is a constitutively active kinase, albeit to a lesser extent than PI3KCAE545K, a hotspot oncogenic mutation. Furthermore, we examined the effectiveness of molecularly targeted PI3K/AKT/mTOR and RAS/MAPK inhibitors to block oncogenic signaling and suppress the growth of OHSU-SARC001 cells. Dual PI3K/mTOR (LY3023414, bimiralisib) and AKT inhibitors (ipatasertib, afuresertib) induced dose-dependent reductions in cell growth. However, mTOR-selective inhibitors (everolimus, rapamycin) alone did not exert cytotoxic effects. The MEK1/2 inhibitor trametinib did not impact proliferation even at the highest doses tested. Our data suggest that molecularly targeted strategies may be effective in PI3K/AKT/mTOR-activated ssRMS. Taken together, these data highlight the importance of utilizing patient-derived models to assess molecularly targetable treatments and their potential as future treatment options.
Keywords: rhabdomyosarcoma.
© 2022 Choo et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Agaram NP, LaQuaglia MP, Alaggio R, Zhang L, Fujisawa Y, Ladanyi M, Wexler LH, Antonescu CR. 2019. MYOD1-mutant spindle cell and sclerosing rhabdomyosarcoma: an aggressive subtype irrespective of age. A reappraisal for molecular classification and risk stratification. Mod Pathol 32: 27–36. 10.1038/s41379-018-0120-9 - DOI - PMC - PubMed
-
- Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, Wan X, Khanna C, Helman LJ. 2008. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res 68: 8039–8048. 10.1158/0008-5472.CAN-08-1712 - DOI - PMC - PubMed
-
- Carlo MI, Molina AM, Lakhman Y, Patil S, Woo K, DeLuca J, Lee CH, Hsieh JJ, Feldman DR, Motzer RJ, et al. 2016. A phase Ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist 21: 787–788. 10.1634/theoncologist.2016-0145 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
