A mass spectrometric method for in-depth profiling of phosphoinositide regioisomers and their disease-associated regulation
- PMID: 35013169
- PMCID: PMC8749000
- DOI: 10.1038/s41467-021-27648-z
A mass spectrometric method for in-depth profiling of phosphoinositide regioisomers and their disease-associated regulation
Abstract
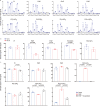
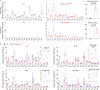
Phosphoinositides are a family of membrane lipids essential for many biological and pathological processes. Due to the existence of multiple phosphoinositide regioisomers and their low intracellular concentrations, profiling these lipids and linking a specific acyl variant to a change in biological state have been difficult. To enable the comprehensive analysis of phosphoinositide phosphorylation status and acyl chain identity, we develop PRMC-MS (Phosphoinositide Regioisomer Measurement by Chiral column chromatography and Mass Spectrometry). Using this method, we reveal a severe skewing in acyl chains in phosphoinositides in Pten-deficient prostate cancer tissues, extracellular mobilization of phosphoinositides upon expression of oncogenic PIK3CA, and a unique profile for exosomal phosphoinositides. Thus, our approach allows characterizing the dynamics of phosphoinositide acyl variants in intracellular and extracellular milieus.
© 2022. The Author(s).
Conflict of interest statement
H.N., J.S., and T.S. have filed for a patent (appl. No. 2018–528909), related to data in this paper on the phosphoinositide measurement method, which is under review. H.N serves as the CEO of and holds equity in LIPIDOME LAB Co., Ltd. T.S. is the founder of Japan Lipid Technologies, LLC. T.Y. serves as the CEO of Japan Lipid Technologies, LLC. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

