Dynamics of spike-and nucleocapsid specific immunity during long-term follow-up and vaccination of SARS-CoV-2 convalescents
- PMID: 35013191
- PMCID: PMC8748966
- DOI: 10.1038/s41467-021-27649-y
Dynamics of spike-and nucleocapsid specific immunity during long-term follow-up and vaccination of SARS-CoV-2 convalescents
Abstract
Anti-viral immunity continuously declines over time after SARS-CoV-2 infection. Here, we characterize the dynamics of anti-viral immunity during long-term follow-up and after BNT162b2 mRNA-vaccination in convalescents after asymptomatic or mild SARS-CoV-2 infection. Virus-specific and virus-neutralizing antibody titers rapidly declined in convalescents over 9 months after infection, whereas virus-specific cytokine-producing polyfunctional T cells persisted, among which IL-2-producing T cells correlated with virus-neutralizing antibody titers. Among convalescents, 5% of individuals failed to mount long-lasting immunity after infection and showed a delayed response to vaccination compared to 1% of naïve vaccinees, but successfully responded to prime/boost vaccination. During the follow-up period, 8% of convalescents showed a selective increase in virus-neutralizing antibody titers without accompanying increased frequencies of circulating SARS-CoV-2-specific T cells. The same convalescents, however, responded to vaccination with simultaneous increase in antibody and T cell immunity revealing the strength of mRNA-vaccination to increase virus-specific immunity in convalescents.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


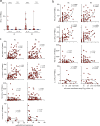


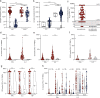

References
Publication types
MeSH terms
Substances
Grants and funding
- Munich site/Deutsches Zentrum für Infektionsforschung (German Center for Infection Research)
- SFB TRR 179/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB TRR 179/Deutsche Forschungsgemeinschaft (German Research Foundation)
- CoVRAPID/Bayerische Forschungsstiftung (Bavarian Research Foundation)
- CoVRapid/Bayerische Forschungsstiftung (Bavarian Research Foundation)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

